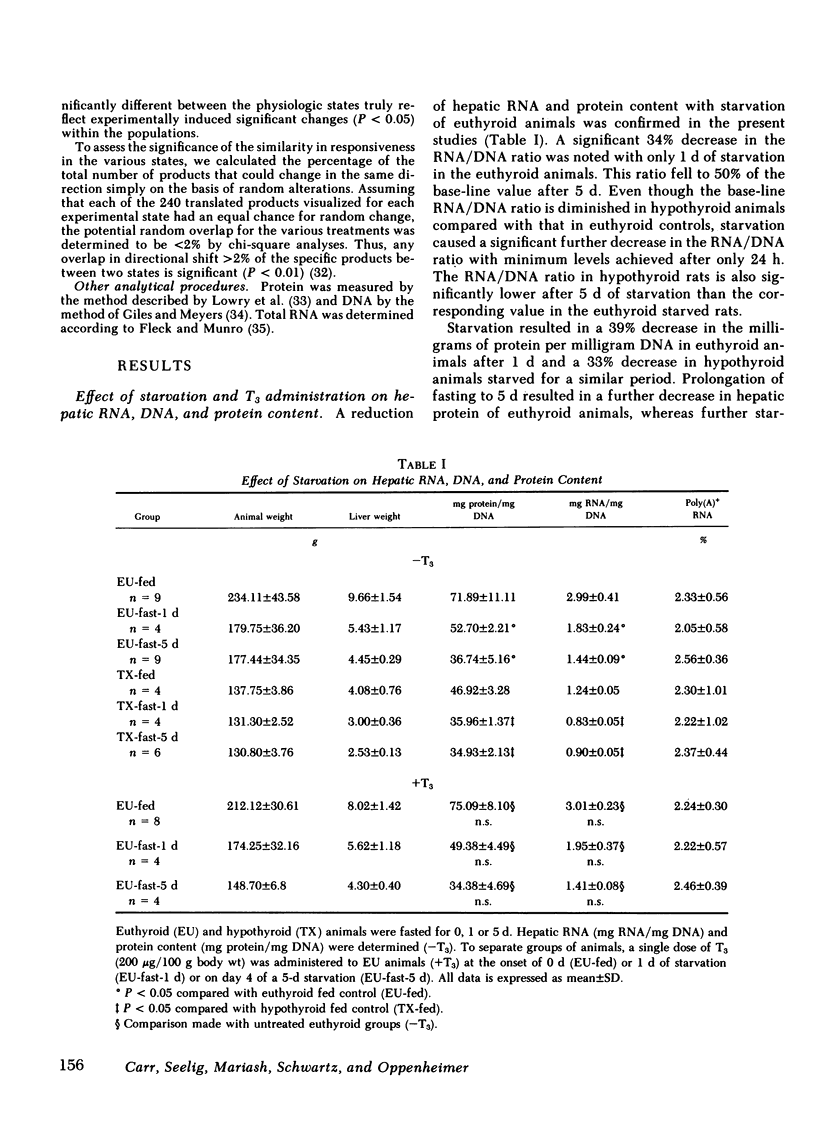
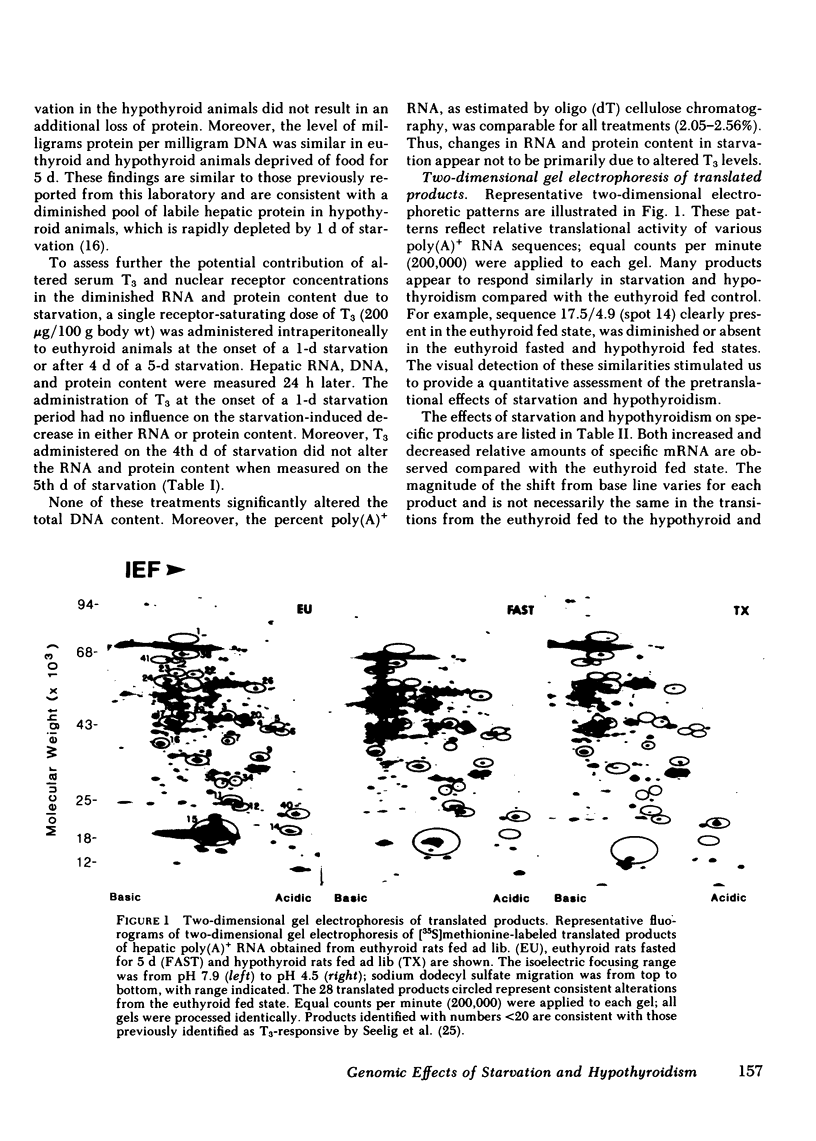
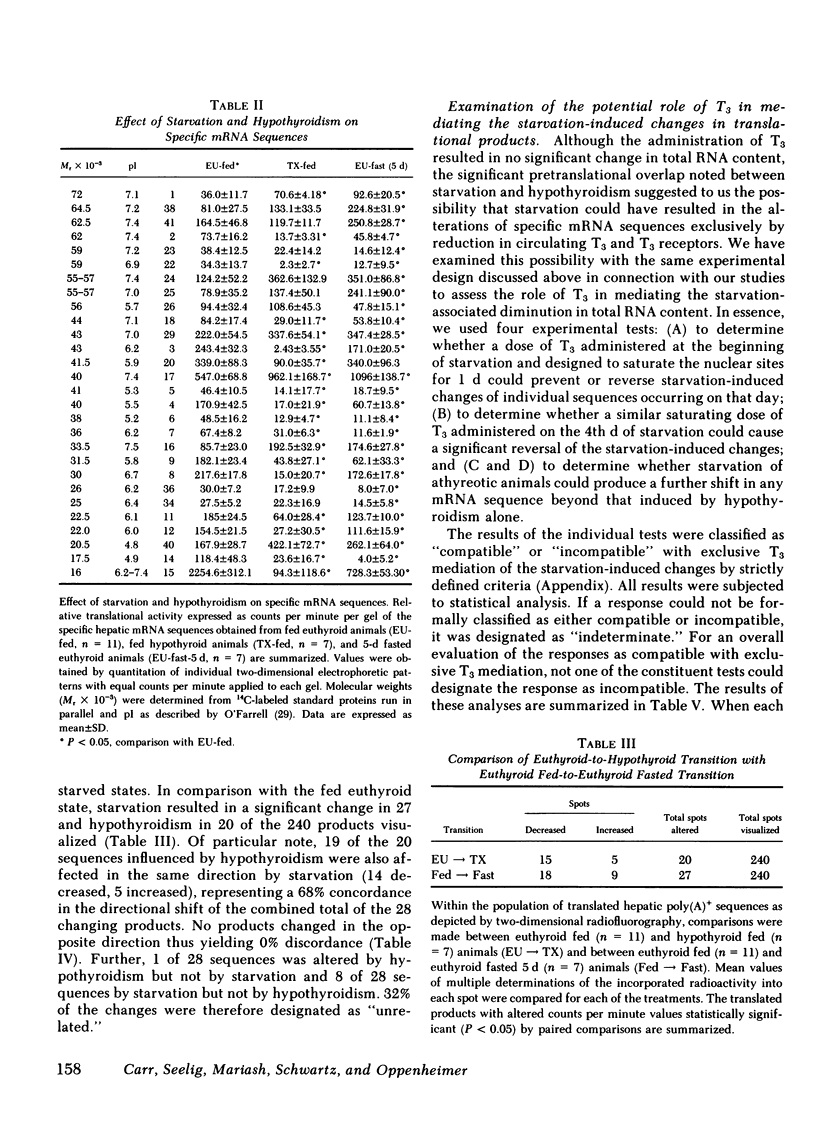
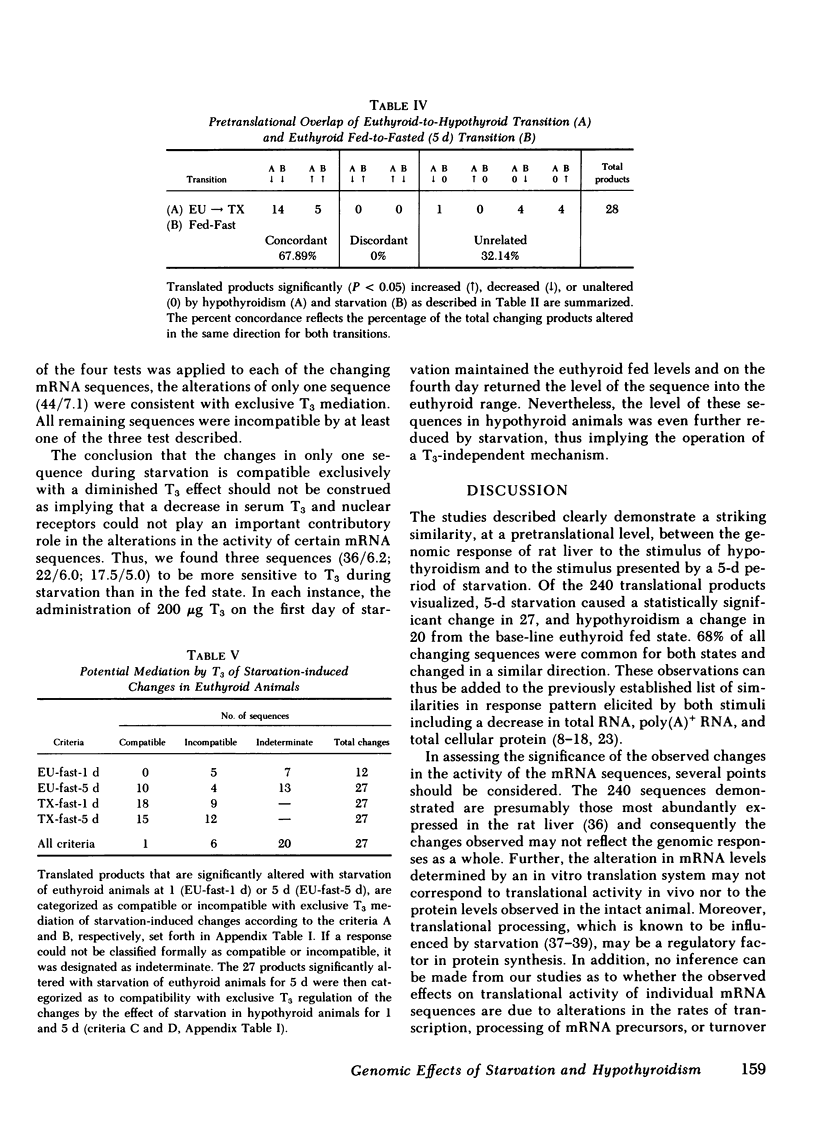
Abstract
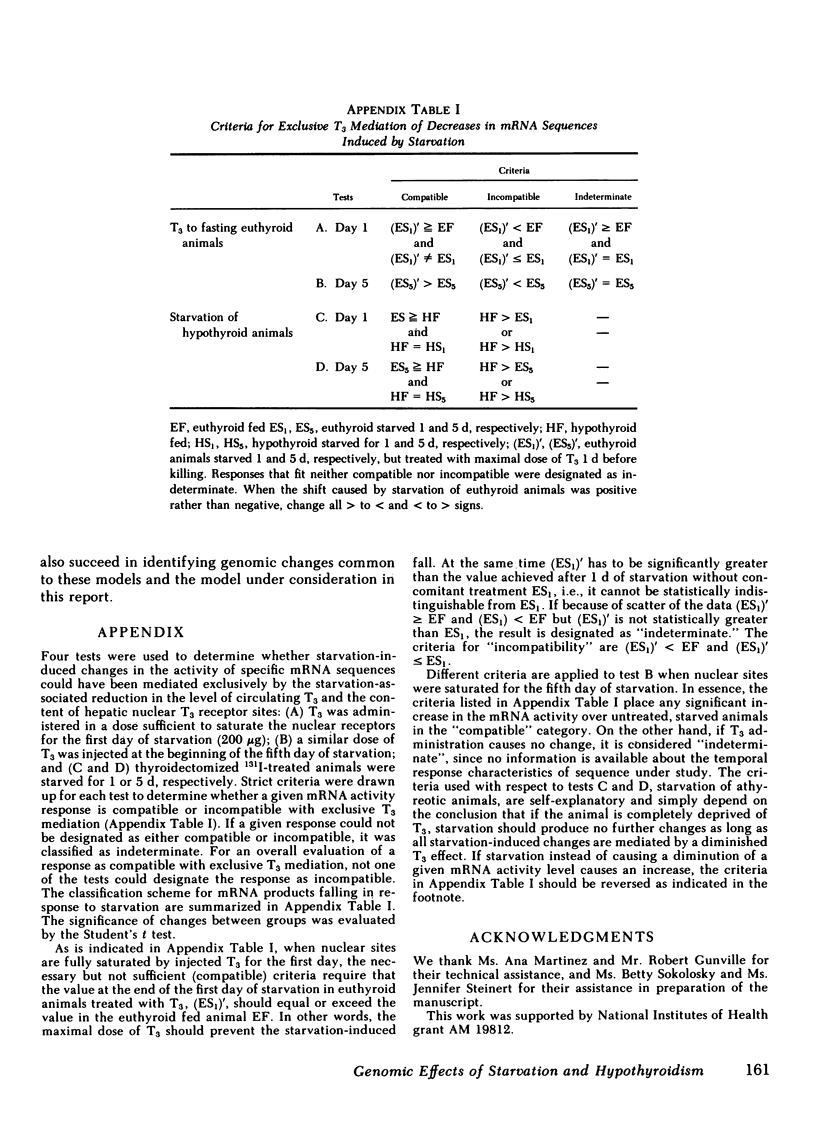
To assess the effect of starvation and to explore the potential interrelationship of starvation and thyroid status at the pretranslational level, we have analyzed by two-dimensional gel electrophoresis, the hepatic translational products of starved and fed euthyroid and hypothyroid rats. 5 d of starvation resulted in a statistically significant change in 27 of 240 products visualized, whereas hypothyroidism caused a change in 20, both in comparison with the fed euthyroid state. Of considerable interest was that 68% of all changing messenger (m)RNA sequences were common to the hypothyroid and starved groups and showed the same directional shift. Further, both starvation and hypothyroidism yielded comparable decreases in total hepatic cytoplasmic RNA content. Although it has been well established that the level of circulating triiodothyronine (T3) and the level of hepatic nuclear receptors fall in starvation, this reduction cannot account for the observed decrease of total hepatic RNA nor for all of the alterations in the concentrations of specific mRNA sequences. Thus, administration of T3 to starved animals in a dose designed to occupy all nuclear T3 receptors fails to prevent the fall in total RNA and the majority of starvation-induced changes in the level of mRNA sequences. Moreover, starvation of athyreotic animals results in a further decrease in total RNA and in a further change in the level of individual mRNA species. We conclude, therefore, that although the reduced levels of circulating T3 and the nuclear T3 receptors can contribute to the observed results of starvation, the starvation-induced changes are not exclusively mediated by this factor. The striking overlap in the genomic response between hypothyroid and starved animals raises the possibility that those biochemical mechanisms regulated at a pretranslational level by T3 are either not helpful or injurious to the starving animal. The reduction in circulating T3 and nuclear receptor sites together with T3-independent mechanisms initiated in the starved animal may constitute redundant processes designed to conserve energy and substrate in the nutritionally deprived organism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsano C. P., Coleoni A. H., DeGroot L. J. Description and partial characterization of thyroid hormone-specific and thyroid hormone-dependent rat liver nuclear proteins. Endocrinology. 1980 May;106(5):1475–1488. doi: 10.1210/endo-106-5-1475. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Dimond R. C., Harvey G. S., O'Brian J. T., Georges L. P., Bruton J., Wright F. D., Wartofsky L. Glucose modulation of alterations in serum iodothyronine concentrations induced by fasting. Metabolism. 1979 Apr;28(4):291–299. doi: 10.1016/0026-0495(79)90098-2. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Lukes Y., Wright F. D., Wartofsky L. Reduction in hepatic triiodothyronine binding capacity induced by fasting. Endocrinology. 1977 Oct;101(4):1331–1334. doi: 10.1210/endo-101-4-1331. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Cottam G. L. Dietary alteration of translatable mRNA sequences coding for rat liver pyruvate kinase. J Biol Chem. 1980 Dec 10;255(23):11499–11503. [PubMed] [Google Scholar]
- Coupar B. E., Davies J. A., Chesterton C. J. Quantification of hepatic transcribing RNA polymerase molecules, polyribonucleotide elongation rates and messenger RNA complexity in fed and fasted rats. Eur J Biochem. 1978 Mar 15;84(2):611–623. doi: 10.1111/j.1432-1033.1978.tb12204.x. [DOI] [PubMed] [Google Scholar]
- DONATI R. M., WARNECKE M. A., GALLAGHER N. I. THE EFFECT OF ABSOLUTE CALORIC DEPRIVATION ON THYROID HORMONE SYNTHESIS AND RELEASE IN THE RAT. Metabolism. 1963 Sep;12:833–836. [PubMed] [Google Scholar]
- DeGroot L. J., Coleoni A. H., Rue P. A., Seo H., Martino E., Refetoff S. Reduced nuclear triiodothyronine receptors in starvation-induced hypothyroidism. Biochem Biophys Res Commun. 1977 Nov 7;79(1):173–178. doi: 10.1016/0006-291x(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H., Oppenheimer J. H. Glucagon influences the expression of thyroid hormone action: discrepancy between nuclear triiodothyronine receptor number and enzyme responses. Endocrinology. 1979 Jul;105(1):74–79. doi: 10.1210/endo-105-1-74. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Schwartz H. L., Oppenheimer J. H. Selective alterations in hepatic enzyme response after reduction of nuclear triiodothyronine receptor sites by partial hepatectomy and starvation. Biochem Biophys Res Commun. 1978 Jan 13;80(1):259–266. doi: 10.1016/0006-291x(78)91131-2. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Distribution of the 9S ribonucleic acid in polysomes of different sizes. Biochemistry. 1969 Mar;8(3):829–831. doi: 10.1021/bi00831a010. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Gardner D. F., Kaplan M. M., Stanley C. A., Utiger R. D. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979 Mar 15;300(11):579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Azizi F., Lipworth L., Vagenakis A. G., Barverman L. E. Effect of starvation on hypothalamic-pituitary-thyroid function in the rat. Metabolism. 1978 Sep;27(9):1074–1083. doi: 10.1016/0026-0495(78)90153-1. [DOI] [PubMed] [Google Scholar]
- Henderson A. R. The effect of feeding with a tryptophan-free amino acid mixture on rat liver magnesium ion-activated deoxyribonucleic acid-dependent ribonucleic acid polymerase. Biochem J. 1970 Nov;120(1):205–214. doi: 10.1042/bj1200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim V. S., Henriquez C., Seo H., Refetoff S., Martino E. Thyroid function in a uremic rat model. Evidence suggesting tissue hypothyroidism. J Clin Invest. 1980 Nov;66(5):946–954. doi: 10.1172/JCI109963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariash C. N., McSwigan C. R., Towle H. C., Schwartz H. L., Oppenheimer J. H. Glucose and triiodothyronine both induce malic enzyme in the rat hepatocyte culture: evidence that triiodothyronine multiplies a primary glucose-generated signal. J Clin Invest. 1981 Dec;68(6):1485–1490. doi: 10.1172/JCI110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L. Factors determining the level of activity of 3,5,3'-triiodothyronine-responsive hepatic enzymes in the starved rat. Endocrinology. 1980 Nov;107(5):1460–1468. doi: 10.1210/endo-107-5-1460. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- RAY R. D., SIMPSON M. E., LI C. H., ASLING C. W., EVANS H. M. Effects of the pituitary growth hormone and thyroxin on growth and differentiation of the skeleton of the rat thyroidectomized at birth. Am J Anat. 1950 May;86(3):479–516. doi: 10.1002/aja.1000860306. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. Effect of starvation of the distribution of free and membrane-bound ribosomes in rat liver and on the content of phospholipid and glycogen in purified ribosomes. Biochim Biophys Acta. 1976 Oct 18;447(3):312–318. doi: 10.1016/0005-2787(76)90054-x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R. L., Maturlo S. J., Surks M. I. Changes in thyroidal economy in rats bearing transplantable Walker 256 carcinomas. Endocrinology. 1980 May;106(5):1386–1391. doi: 10.1210/endo-106-5-1386. [DOI] [PubMed] [Google Scholar]
- Savage M. J., Sala-Trepat J. M., Bonner J. Measurement of the complexity and diversity of poly(adenylic acid) containing messenger RNA from rat liver. Biochemistry. 1978 Feb 7;17(3):462–467. doi: 10.1021/bi00596a014. [DOI] [PubMed] [Google Scholar]
- Schussler G. C., Orlando J. Fasting decreases triiodothyronine receptor capacity. Science. 1978 Feb 10;199(4329):686–688. doi: 10.1126/science.204004. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Lancer S. R., Oppenheimer J. H. Thyroid hormones influence starvation-induced hepatic protein loss in the rat: possible role of thyroid hormones in the generation of labile protein. Endocrinology. 1980 Dec;107(6):1684–1692. doi: 10.1210/endo-107-6-1684. [DOI] [PubMed] [Google Scholar]
- Seelig S., Liaw C., Towle H. C., Oppenheimer J. H. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4733–4737. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui U. A., Goldflam T., Goodridge A. G. Nutritional and hormonal regulation of the translatable levels of malic enzyme and albumin mRNAs in avian liver cells in vivo and in culture. J Biol Chem. 1981 May 10;256(9):4544–4550. [PubMed] [Google Scholar]
- Sonenshein G. E., Brawerman G. Entry of mRNA into polyribosomes during recovery from starvation in mouse sarcoma 180 cells. Eur J Biochem. 1977 Feb 15;73(1):307–312. doi: 10.1111/j.1432-1033.1977.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Suda A. K., Pittman C. S., Shimizu T., Chambers J. B., Jr The production and metabolism of 3,5,3'-triiodothyronine and 3,3',5-triiodothyronine in normal and fasting subjects. J Clin Endocrinol Metab. 1978 Dec;47(6):1311–1319. doi: 10.1210/jcem-47-6-1311. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Dillmann W. H., Oppenheimer J. H. Messenger RNA content and complexity of euthyroid and hypothyroid rat liver. J Biol Chem. 1979 Apr 10;254(7):2250–2257. [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Schwartz H. L., Oppenheimer J. H. Quantitation of rat liver messenger ribonucleic acid for malic enzyme during induction by thyroid hormone. Biochemistry. 1981 Jun 9;20(12):3486–3492. doi: 10.1021/bi00515a028. [DOI] [PubMed] [Google Scholar]
- Vignati L., Finley R. J., Hagg S., Aoki T. T. Protein conservation during prolonged fast: a function of triiodothyronine levels. Trans Assoc Am Physicians. 1978;91:169–179. [PubMed] [Google Scholar]
- Wimpfheimer C., Saville E., Voirol M. J., Danforth E., Jr, Burger A. G. Starvation-induced decreased sensitivity of resting metabolic rate to triiodothyronine. Science. 1979 Sep 21;205(4412):1272–1273. doi: 10.1126/science.224460. [DOI] [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Effect of a short term fast on the distribution of cytoplasmic albumin messenger ribonucleic acid in rat liver. Evidence for formation of free albumin messenger ribonucleoprotein particles. J Biol Chem. 1978 Jul 25;253(14):4944–4950. [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Identification of albumin mRNPs in the cytosol of fasting rat liver and influence of tryptophan or a mixture of amino acids. Biochem Biophys Res Commun. 1978 Jul 28;83(2):427–433. doi: 10.1016/0006-291x(78)91008-2. [DOI] [PubMed] [Google Scholar]



