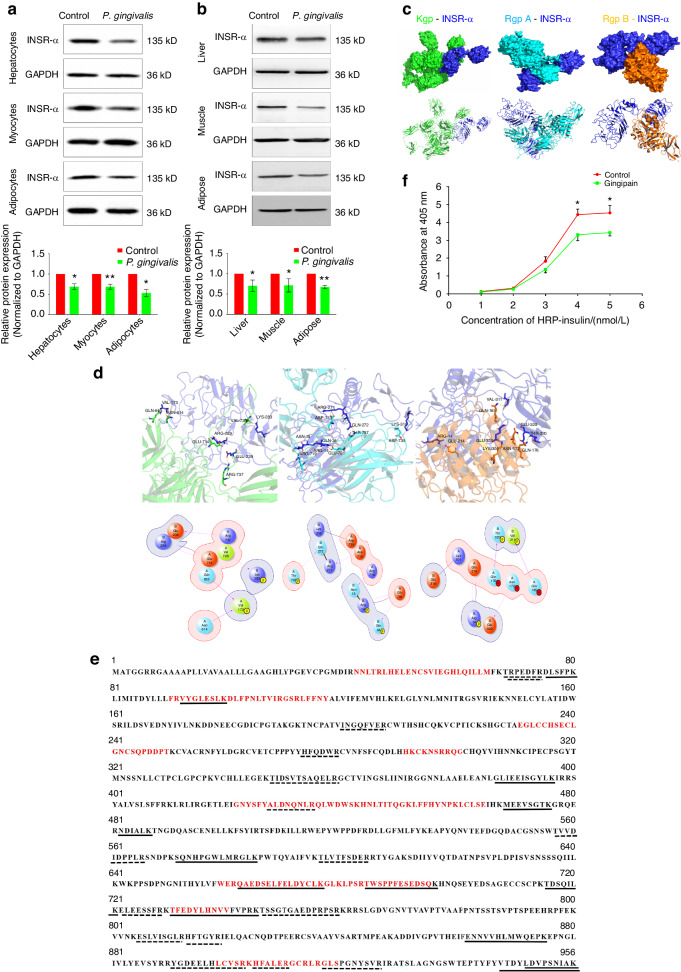
Fig. 7.
Gingipain can directly hydrolyse the INSR α subunit and affect its binding to insulin. a Changes in the protein expression of INSR in hepatocytes, myocytes and adipocytes after coculture with P. gingivalis and semiquantitative analysis of INSR (the expression intensity of proteins in the control group was set as 1). b Changes in the protein expression of the INSR α subunit in liver, skeletal muscle and adipose tissues after coculture with P. gingivalis and semiquantitative analysis of the INSR α subunit (the expression intensity of proteins in the control group was set as 1). c Molecular docking analysis between INSR and gingipain. Upper: surface pattern diagram; lower: ribbon pattern diagram. d Molecular docking analysis of the interaction between INSR and gingipain. Upper: 3D pattern diagram; lower: 2D pattern diagram. e LC‒MS/MS analysis of hydrolysis fragments of INSR after Kgp (solid lines) or RgpA/B (dotted lines) incubation; red amino acids indicated functional binding region of INSR. f ELISA of INSR–insulin binding ability changes after the effect of gingipain. INSR-α, insulin receptor α subunit; HRP, horseradish peroxidase; *P < 0.05; **P < 0.01