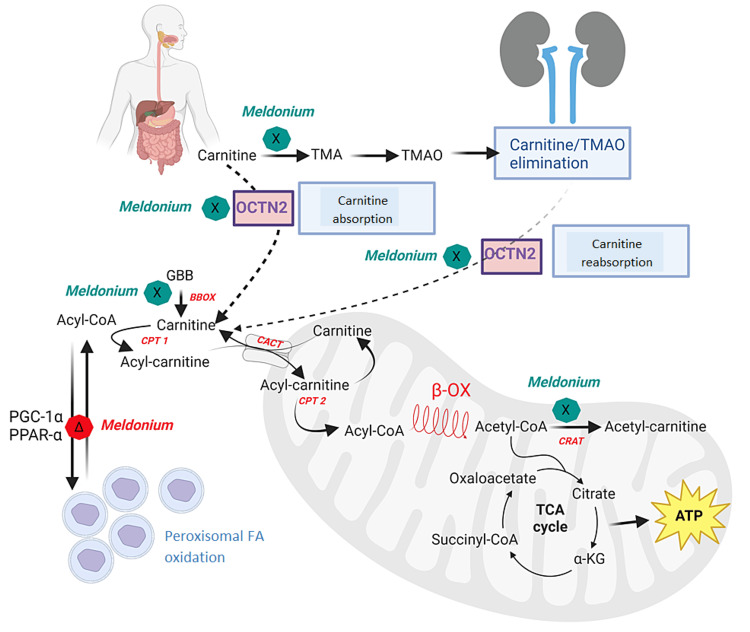
Figure 2. Meldonium mechanism of action.
In the cytoplasm, the activated fatty acid as acyl-CoA binds to L-carnitine, forming acyl-carnitine by carnitine palmitoyltransferase-1 (CPT1). Acyl-carnitine is transferred into the mitochondria under the action of carnitine/acyl-carnitine translocase (CACT). Inside the mitochondria, acyl-carnitine is converted back to acyl-CoA and L-carnitine by the action of carnitine palmitoyltransferase-2 (CPT2) and enters the process of β-oxidation (β-OX) to form acetyl-CoA. Acetyl-CoA is further metabolized in the Krebs cycle or transformed into acetyl-carnitine by the enzyme carnitine acetyltransferase (CrAT). Within the mitochondria, meldonium inhibits the CrAT enzyme, thus regulating the ratio of acetyl-CoA/acetyl-carnitine. By inhibiting the enzyme γ-butyrobetaine hydroxylase (BBOX), meldonium inhibits the synthesis of L-carnitine at the final step, the transformation of γ-butyrobetaine (GBB) into L-carnitine, thereby reducing its concentration in the cell. Fatty acids are redirected to peroxisomes where they are transported and oxidized independently of L-carnitine.
Meldonium induces increased gene expression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and peroxisome proliferator-activated receptor alpha (PPAR-α). At the intestinal level, meldonium inhibits organic cation/carnitine transporter type 2 (OCTN2) and decreases the absorption of dietary L-carnitine into the body, which is then eliminated through urine. Additionally, meldonium inhibits the formation of trimethylamine (TMA) from GBB by intestinal microbiota and promotes the elimination of trimethylamine-N-oxide (TMAO) through urine. At the renal level, meldonium reduces the reabsorption of L-carnitine. Green marks signify inhibition and red marks signify activation of a pathway.
Source: This image is the original work of the authors, and the image was created by BioRender.com.