ABSTRACT
Introduction
The DAPA-CKD study showed a protective effect of dapagliflozin on kidney function in chronic kidney disease (CKD) patients with and without diabetes mellitus. Although dapagliflozin is expected to be effective also in CKD patients with autosomal dominant polycystic kidney disease (ADPKD), its efficacy and safety in this population remain unknown because ADPKD was an exclusion criterion in the DAPA-CKD study. Therefore, we evaluated the effects of dapagliflozin in CKD patients with ADPKD.
Methods
We performed a retrospective observational study of seven patients with ADPKD treated with dapagliflozin at Toranomon Hospital, Tokyo, Japan. We analyzed changes in estimated glomerular filtration rate (eGFR) slope and annual height-corrected total kidney volume before and after starting dapagliflozin treatment.
Results
The median observation period after starting dapagliflozin was 20 months. Four patients received concomitant tolvaptan. The eGFR slope before and after initiation of dapagliflozin could be calculated in six patients and improved in all of them except the one who did not receive a renin-angiotensin system (RAS) inhibitor. Annual height-corrected total kidney volume increased in all patients. Concurrent tolvaptan treatment had no effect.
Conclusion
In CKD patients with ADPKD, dapagliflozin may increase kidney volume but may have a protective effect on kidney function when used concomitantly with RAS inhibitors.
Keywords: ADPKD, CKD, dapagliflozin, eGFR slope, total kidney volume
KEY LEARNING POINTS.
What was known:
Animal studies have shown that in ADPKD, SGLT2 inhibitors are expected to exert protective effects on the kidneys by reducing blood pressure, facilitating weight loss, and altering metabolism, cell signaling, and autophagy. However, some authors have expressed concerns about the increase in kidney volume due to increased antidiuretic hormone (ADH) secretion and urine osmolality.
This study adds:
The eGFR slope before and after initiation of dapagliflozin could be calculated in six patients and improved in all of them except the one who did not receive a renin-angiotensin system (RAS) inhibitor. Annual height-corrected total kidney volume increased in all patients.
Potential impact:
In humans with ADPKD, dapagliflozin may increase kidney volume but may have a protective effect on kidney function when used concomitantly with RAS inhibitors.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease and is present in ∼3.7% of dialysis patients (∼12 500 patients) in Japan. Traditionally, about half of the patients with ADPKD were reported to develop end-stage kidney disease by the age of 60, but findings indicate that since the introduction of tolvaptan in Japan in 2014 as a treatment covered by public health insurance, on average patients are older before they reach end-stage kidney disease. However, some patients cannot be treated with tolvaptan because of adverse effects of the diuresis of 4 to 5 l or more per day or because they develop liver dysfunction; therefore, new therapeutic agents are required.
In 2020, the DAPA-CKD (Dapagliflozin in Patients with Chronic Kidney Disease) study demonstrated a protective effect of the sodium-glucose cotransporter-2 (SGLT2) inhibitor dapagliflozin on kidney function in chronic kidney disease (CKD) patients with and without diabetes mellitus [1]. Subsequently, in 2023, the EMPA-KIDNEY (Empagliflozin in Patients with Chronic Kidney Disease) trial showed a similar effect of empagliflozin, another SGLT2 inhibitor [2]. Therefore, the protective effect of SGLT2 inhibitors on kidney function in CKD patients has attracted attention.
In Japan, public health insurance started to cover dapagliflozin for CKD patients, including patients with CKD associated with ADPKD, in August 2021. However, the efficacy and safety of dapagliflozin in ADPKD is unclear because the clinical trials on dapagliflozin excluded patients with ADPKD, as well as those with antineutrophil cytoplasmic antibody-associated vasculitis and other diseases that cause rapid progression of kidney dysfunction.
In ADPKD, SGLT2 inhibitors are expected to exert protective effects on the kidneys by reducing blood pressure, facilitating weight loss, and altering metabolism, cell signaling, and autophagy [3]. However, some authors have expressed concerns about the increase in kidney volume due to increased ADH secretion and urine osmolality in patients with ADPKD who take these medications [4].
Animal studies on dapagliflozin have reported varying results. For example, in Han: SPRD rats, dapagliflozin improved kidney function and albuminuria but had no effect on cyst size [5]. On the other hand, in PCK rats, dapagliflozin worsened kidney function and albuminuria—possibly because of hyperfiltration—and increased the size of kidney cysts [6]. Furthermore, translation of findings from animal experiments to humans is challenging.
One clinical study evaluated the effects of dapagliflozin in CKD patients with ADPKD and found that it may have exacerbated the decline in kidney function and further increased kidney volume; however, the observation period was short, averaging 108 days, and it is possible that the effect of the initial dip was still present [7]. Therefore, the present study examined how dapagliflozin treatment affects kidney function and volume in ADPKD patients over a longer period.
MATERIALS AND METHODS
Patients
This retrospective study included seven patients with ADPKD treated with dapagliflozin between January 2014 and May 2023 at Toranomon Hospital, Tokyo, Japan. Eligibility criteria included a diagnosis of ADPKD according to the modified Pei–Ravine criteria from the protocol of the REPRISE (Replicating Evidence of Preserved Kidney Function: An Investigation of Tolvaptan Safety and Efficacy in ADPKD) trial [8, 9, 10].
The study was performed in accordance with the Declaration of Helsinki and was approved by the research ethics committees of Toranomon Hospital. The requirement for informed consent was waived because of the retrospective nature of the study.
Kidney function
eGFR was calculated with the Japanese eGFR equation [11]. The annual change in eGFR before and after dapagliflozin administration (Δ eGFR slope) was defined as the difference between the annual change in eGFR before initiation of dapagliflozin treatment (pre-eGFR slope) and the annual change in eGFR after initiation of treatment (post-eGFR slope), i.e. post-eGFR slope—pre-eGFR slope. The eGFR slope was calculated using the least-squares methods, assuming that the eGFR trajectory is linear. Considering the assumption that there is an initial dip, the slope was not calculated within 7 weeks after starting or stopping tolvaptan and 15 weeks after starting dapagliflozin (Supplementary methods). Missing data were defined as those in which eGFR could not be continuously measured for >6 months before and after the introduction of dapagliflozin.
Initial dip
If eGFR decreased within 9 weeks after starting dapagliflozin treatment, the initial dip was quantified as the percentage decrease from the eGFR when starting dapagliflozin to the lowest eGFR within this 9-week period.
Total kidney volume
Total kidney volume (TKV) was estimated from computed tomography scans or magnetic resonance images by volume measurement software (Synapse Vincent, Fujifilm, Japan). TKV divided by height (m) was used as the height-adjusted TKV (htTKV). The change in the annual increase in htTKV before and after the start of dapagliflozin treatment (Δ%ΔhtTKV/y) was calculated from the difference in the annual htTKV increase before starting dapagliflozin treatment (pre-%ΔhtTKV/y) and afterwards (post-%ΔhtTKV/y), i.e. as post-%ΔhtTKV/y − pre-%ΔhtTKV/y. Data were not included if the total measurement period of hTKV was shorter than 6 months before and after dapagliflozin's introduction (Supplementary methods).
Statistical analysis
Comparisons of ΔeGFR slopes and Δ%ΔhtTKV values before and after initiation of dapagliflozin treatment were performed by Wilcoxon signed rank test. For all analyses, a P value of <0.05 was taken to indicate statistical significance. All analyses were performed with STATA® SE version 18.0 (StataCorp, College Station, TX, USA).
RESULTS
Clinical and laboratory characteristics
A summary of the background and clinical data of all patients is presented in Table 1. The seven patients comprised five men and two women, and the median observation period after initiation of dapagliflozin treatment was 20 months. Four patients were treated with tolvaptan. All patients were diabetes-free and were hypertensive. At the start of dapagliflozin treatment, all measurements of urinary protein were less than 0.5 g/gCre in six patients, and one patient had a negative qualitative urinary protein test, although quantitative data were not available. At the start of dapagliflozin, the median eGFR was 26.9 ml/min/1.73 m2 (interquartile range, 22.6–43.1 ml/min/1.73 m2), and the median htTKV, 1444 ml/m (interquartile range, 517−2320 ml/m).
Table 1:
Clinical characteristics of CKD patients with ADPKD starting treatment with dapagliflozin.
| Patient | |||||||
|---|---|---|---|---|---|---|---|
| Sex | F | F | M | M | M | M | M |
| Age | 65 | 52 | 67 | 51 | 37 | 54 | 44 |
| Hypertension | + | + | + | + | + | + | + |
| Diabetes mellitus | − | − | − | − | − | − | − |
| RAS inhibitor | − | + | + | + | + | + | + |
| Tolvaptan | + | + | + | − | + | − | − |
| DAPA (mg/day) | 10 | 5 | 10 | 5* | 5 | 10 | 10 |
| DAPA dosing period, months | 22 | 13 | 22 | 21 | 20 | 17 | 14 |
| eGFR (ml/min/1.73 m2) | 22.6 | 21.8 | 26.9 | 44.8 | 38.5 | 43.1 | 23.3 |
| Urinary protein (g/gCre) | 00.16 | 00.13 | 00.25 | 00.04 | NA | 00.4 | 00.29 |
| htTKV (ml/m) | 517 | 637 | 2402 | 509 | 2320 | 1680 | 1444 |
Cre, creatinine; DAPA, dapagliflozin; eGFR, estimated glomerular filtration rate; F, female; htTKV, height-adjusted TKV; M, male; NA, not available; RAS, renin-angiotensin system inhibitors
* In this patient, the dose was increased to 10 mg/day 14 months after initiation of dapagliflozin treatment.
Initial dip
Among the seven patients, five had an initial dip and two did not. Of the five patients with an initial dip, the dip exceeded 10% in three and was 4.87% in one.
Change in eGFR slope (ΔeGFR slope)
The eGFR slope before and after dapagliflozin treatment could be calculated in six patients and improved in five of them after the start of dapagliflozin treatment (Table 2; Fig. 1; P = 0.12). We calculated the eGFR slope from the median (interquartile range) number of creatinine measurements before and after dapagliflozin administration, i.e. 27 (14.5–58.25) and 12.5 (8.25–16.75), respectively.
Table 2:
Annual change in estimated glomerular filtration rate before and after dapagliflozin administration and initial dip in estimated glomerular filtration rate after introduction of dapagliflozin.
| Patient | eGFR (ml/min/1.73 m2) | Pre-eGFR slope (ml/min/1.73 m2/year) | Post-eGFR slope (ml/min/1.73 m2/year) | ΔeGFR slope (ml/min/1.73 m2/year) | Initial dip in eGFR (%) |
|---|---|---|---|---|---|
| 1 | 22.6 | −1.1 | −2.3 | −1.2 | 22.7 |
| 2 | 21.8 | −1.9 | −0.6 | 1.3 | 12.2 |
| 3 | 26.9 | NA | −0.1 | NA | 12.9 |
| 4 | 44.8 | −2.6 | −0.8 | 1.8 | 19.6 |
| 5 | 38.5 | −3.3 | −1.9 | 1.4 | 0 |
| 6 | 43.1 | −3.0 | −1.9 | 1.1 | 0 |
| 7 | 23.3 | −2.9 | −2.8 | 0.1 | 4.9 |
pre-eGFR slope, annual change in eGFR before dapagliflozin initiation; post-eGFR slope, annual change in eGFR after dapagliflozin initiation; ΔeGFR slope, post-eGFR slope − pre-eGFR slope
Figure 1:
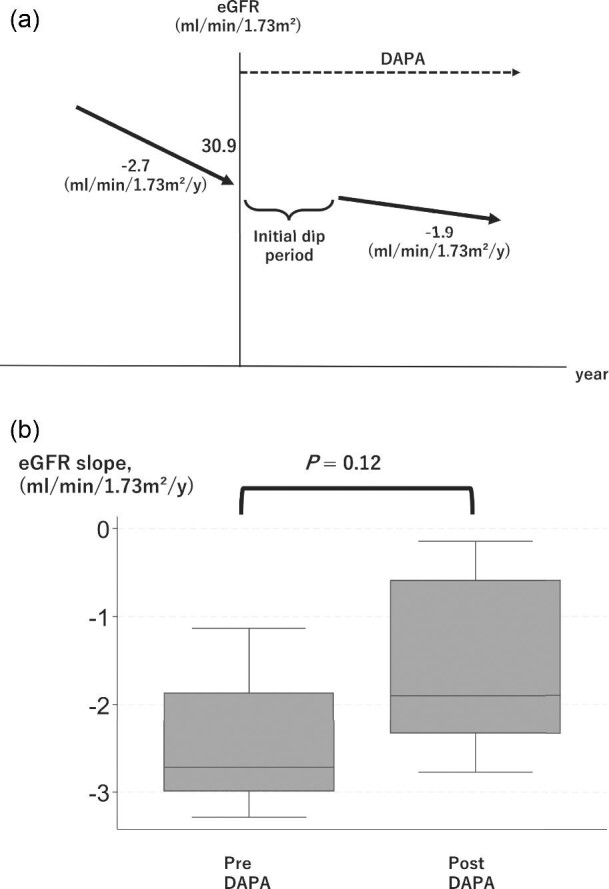
(a) Imaged figure of TKV trajectory drawn from the median. (b) Box plot of change in the slope of the estimated glomerular filtration rate before and after the start of dapagliflozin treatment.
The eGFR slope worsened in only one patient, and this was the only patient who was not taking RAS inhibitor. When this patient was excluded from the analysis, the eGFR slope showed significant improvement after the start of dapagliflozin (Supplementary Figure; P = 0.04). Furthermore, a positive correlation (correlation coefficient, 0.66) was found between the initial eGFR and the ΔeGFR slope, indicating that a higher initial eGFR corresponded to a more pronounced improvement in the eGFR slope.
There was no significant difference in ΔeGFR slope between patients with and without tolvaptan.
Change in %ΔhtTKV/y(Δ%ΔhtTKV/y)
The annual change in htTKV (%ΔhtTKV/y) from before to after the start of dapagliflozin treatment could be calculated in five patients and increased in all of them (Table 3; Fig. 2; P = 0.04). Furthermore, a strong positive correlation (correlation coefficient, 0.94) was observed between htTKV at treatment onset and Δ%ΔhtTKV/y, indicating that a greater initial htTKV corresponded to a more pronounced rate of enlargement. There was no significant difference in ΔhtTKV/y between patients with and without tolvaptan.
Table 3:
Annual percentage increase and change before and after dapagliflozin administration in height-adjusted TKV.
| Patient | htTKV (ml/m) | Pre-%ΔhtTKV/y (%) | Post-%ΔhtTKV/y (%) | Δ%ΔhtTKV/y (%) |
|---|---|---|---|---|
| 1 | 517 | 0.9 | 11.0 | 10.1 |
| 2 | 637 | 13.0 | NA | NA |
| 3 | 2402 | −7.5 | 19.3 | 26.8 |
| 4 | 509 | 4.6 | 8.2 | 3.6 |
| 5 | 2320 | 1.0 | NA | NA |
| 6 | 1680 | −0.8 | 15.0 | 15.8 |
| 7 | 1444 | 10.9 | 23.6 | 12.7 |
Pre-%ΔhtTKV, percentage increase in height-adjusted TKV per year before dapagliflozin initiation; post-%ΔhtTKV, percentage increase in height-adjusted TKV per year after dapagliflozin initiation; Δ%ΔhtTKV, post-%ΔhtTKV − pre-%ΔhtTKV
Figure 2:
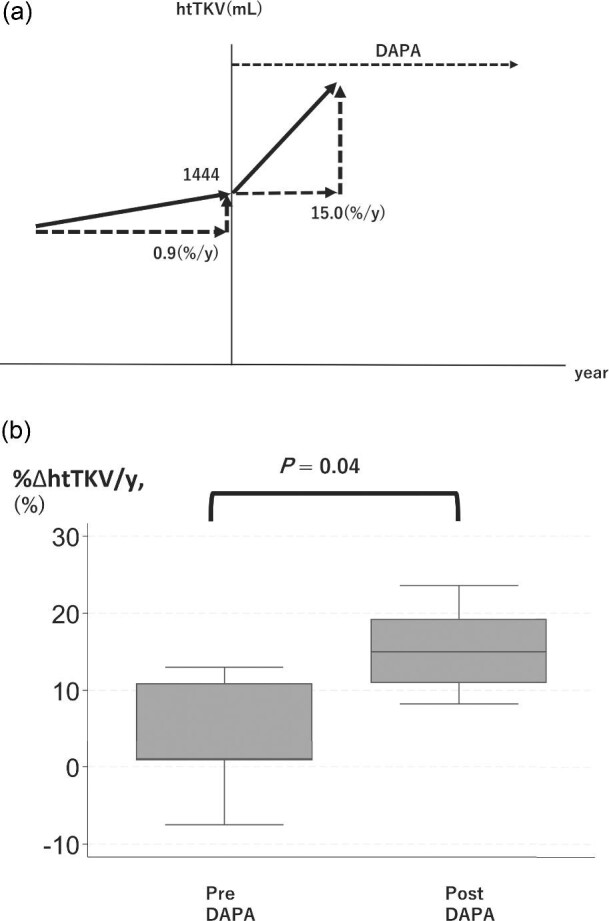
(a) Imaged figure of TKV trajectory drawn from the median. (b) Box plot of the slope of the annual change in height-adjusted TKV before and after the start of dapagliflozin treatment %ΔhtTKV/y, annual change in height-adjusted TKV; DAPA, dapagliflozin.
Cyst infections
No patient had a cyst infection during the observation period.
DISCUSSION
To our knowledge, this is the first time that the effects of dapagliflozin in several ADPKD patients have been evaluated in a study with an observation period of >1 year. The comparison of the eGFR slope before and after the start of dapagliflozin treatment in the six patients for whom the slope could be calculated found that it improved in all patients except the one who was not taking an RAS inhibitor, and in all five patients for whom %ΔhtTKV/y could be calculated, %ΔhtTKV/y showed an increasing trend.
Two case reports have described the use of dapagliflozin in patients with ADPKD, and the findings of the reports were consistent with our study, i.e. one showed an improvement in the eGFR slope [12], and the other showed an increase in kidney volume [13].
In ADPKD patients with significant kidney volumes, the progression of kidney function decline is usually rapid [14]. However, in the present study, dapagliflozin suppressed kidney function decline even though it contributed to increased kidney volume.
Dapagliflozin contributes to kidney protection by decreasing intraglomerular pressure through restoration of tubulo-glomerular feedback, and the initial dip reflects this mechanism [15, 16, 17]. In the present study, dapagliflozin treatment has protective effect of on kidney function in all patients except one who did not receive an RAS inhibitor. Since dapagliflozin caused an initial dip in some patients, it is considered that ADPKD patients may also benefit from the protective effects on kidney function associated with the correction of hyperfiltration.
As mentioned before, the eGFR slope worsened with dapagliflozin treatment only in the patient who was not treated with an RAS inhibitor. The DAPA-CKD study was designed to evaluate the efficacy of dapagliflozin in combination with RAS inhibitors [1], so almost all patients in both the dapagliflozin and the control groups were treated with RAS inhibitors; because of the paucity of data, protective effect of dapagliflozin on kidney function in the absence of concomitant RAS inhibitors is not apparent. In ADPKD patients with concomitant hypertension, the RAS system is often considered to be hyperactive [18], and the decrease in fluid volume associated with dapagliflozin treatment may cause further activation of the RAS system, leading to the progression of kidney function decline.
In all patients, the annual change in kidney volume increased with dapagliflozin use. In general, kidney volume enlargement and kidney function decline are believed to progress concurrently in patients with ADPKD, so the deviation from this conventional notion is a significant finding of the present study. In experiments with wild-type mice, empagliflozin was reported to increase kidney weight, and dilatation of the proximal tubular S3 segment and collecting ducts was observed [19]. Glucose reabsorption via the SGLT1 cotransporter in the S3 segment was shown to increase when SGLT2 inhibitors were administered [20]. Cystic enlargement in ADPKD is due to increased glucose uptake [21, 22], so increased glucose uptake in the S3 segment after administration of dapagliflozin may be responsible for the increase in cyst size after treatment initiation. As for the dilation of the collecting ducts, previous research observed elevated serum vasopressin and increased expression of aquaporin-2 (Aqp-2) and Aqp-3 with empagliflozin, suggesting that cell proliferation and increased cystic fluid secretion via cyclic adenosine monophosphate proliferation may be responsible for cystic enlargement [19]. In addition, the study compared the expression of hypoxia-inducible factor-1 alpha (HIF-1α) throughout the tubules with that in controls and confirmed that expression was increased almost exclusively in the collecting duct cells [19]. In another study, anoctamin1 and P2Y2R were upregulated in mice overexpressing HIF-1α, and the increased cystic fluid secretion mediated by these proteins was suggested to contribute to cystic enlargement [23]. As described previously, in ADPKD dapagliflozin causes activation of the V2 receptor signaling pathway and overexpression of HIF-1α, which may lead to cyst enlargement in the collecting duct. Although tolvaptan suppresses cyst enlargement via V2 receptor antagonism, in the present study cyst enlargement was also observed in patients receiving concomitant tolvaptan.
Dapagliflozin has a protective effect on the kidney in that it decreases intraglomerular hyperfiltration, and the benefit for kidney function may be greater than the negative effect of increasing kidney volume. This overall protective effect on the kidney may explain why the eGFR slope improved in the present study despite the increase in kidney volume. However, it remains to be determined whether the protective effect on the kidney is maintained with longer-term use of dapagliflozin.
The current study has some limitations. First, it was a single-center, retrospective study with a small sample size and no control group, which may limit the generalizability of the data because ADPKD is influenced by genetic and environmental factors and therefore has a highly heterogeneous course. Second, it may have a selection bias because we included only patients treated with dapagliflozin. Third, the measurement period of htTKV is not standardized, so comparing annual growth rates may not be accurate.
Despite these limitations, our finding that dapagliflozin may improve kidney function but also promote kidney enlargement is of interest and should be further examined in a large, prospective, multicenter study.
In conclusion, dapagliflozin treatment of CKD patients with ADPKD may be effective in preventing kidney function decline in patients with concomitant use of RAS inhibitors. On the other hand, it may increase kidney volume, with a tendency toward a greater increase in patients with larger kidney volumes. Dapagliflozin should be introduced with care in patients with ADPKD, and patients should be closely monitored.
Supplementary Material
ACKNOWLEDGEMENTS
The manuscript was edited by a native English-speaking medical editor from Yamada Translation Bureau, Inc. (Tokyo, Japan).
Contributor Information
Masatoshi Yoshimoto, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Akinari Sekine, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
Tatsuya Suwabe, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
Yuki Oba, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Hiroki Mizuno, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Masayuki Yamanouchi, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
Yoshifumi Ubara, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Junichi Hoshino, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Department of Nephrology, Tokyo Women's Medical University, Tokyo, Japan.
Noriko Inoue, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Kiho Tanaka, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Eiko Hasegawa, Nephrology Center, Toranomon Hospital, Tokyo, Japan.
Naoki Sawa, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
Takehiko Wada, Nephrology Center, Toranomon Hospital, Tokyo, Japan; Okinaka Memorial Institute for Medical Research, Tokyo, Japan.
FUNDING
This work was supported by a research grant from the Okinaka Memorial Institute to A.S, in part by JSPS KAKENHI Grant Number 22K08321 to T.W., and in part by a Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan to T.W.
None of the funding bodies had any role in the study design, data analysis, decision to publish, or preparation of the manuscript.
AUTHORS’ CONTRIBUTIONS
M.Y., A.S., and T.W. designed the study; M.Y., A.S., T.S., N.S., Y.U., and T.W. managed the patients and collected the clinical data; M.Y. and A.S. prepared the figures and tables; and all authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
STATEMENT OF ETHICS
This study was performed in accordance with the Declaration of Helsinki and was approved by the research ethics committees of Toranomon Hospital (Approval Number 2473-H/B).
REFERENCES
- 1. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 2. Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afsar B, Afsar RE, Demiray A et al. Sodium-glucose cotransporter inhibition in polycystic kidney disease: fact or fiction. Clin Kidney J 2022;15:1275–83. 10.1093/ckj/sfac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel DM, Dahl NK. Examining the role of novel CKD therapies for the ADPKD patient. Kidney360 2021;2:1036–41. 10.34067/KID.0007422020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez D, Kapoor S, Edenhofer I et al. Inhibition of sodium-glucose cotransporter 2 with dapagliflozin in Han: SPRD rats with polycystic kidney disease. Kidney Blood Press Res 2015;40:638–47. 10.1159/000368540 [DOI] [PubMed] [Google Scholar]
- 6. Kapoor S, Rodriguez D, Riwanto M et al. Effect of sodium-glucose cotransport inhibition on polycystic kidney disease progression in PCK rats. PLoS ONE 2015;10:e0125603. 10.1371/journal.pone.0125603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morioka F, Nakatani S, Uedono H et al. Short-term dapagliflozin administration in autosomal dominant polycystic kidney disease-a retrospective single-arm case series study. JCM 2023;12. 10.3390/jcm12196341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres VE, Devuyst O, Chapman AB et al. Rationale and design of a clinical trial investigating tolvaptan safety and efficacy in autosomal dominant polycystic kidney disease. Am J Nephrol 2017;45:257–66. 10.1159/000456087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pei Y, Obaji J, Dupuis A et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol: JASN 2009;20:205–12. 10.1681/ASN.2008050507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ravine D, Gibson RN, Walker RG et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet North Am Ed 1994;343:824–7. 10.1016/S0140-6736(94)92026-5 [DOI] [PubMed] [Google Scholar]
- 11. Matsuo S, Imai E, Horio M et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 12. Minatoguchi S, Hayashi H, Umeda R et al. Additional renoprotective effect of the SGLT2 inhibitor dapagliflozin in a patient with ADPKD receiving tolvaptan treatment. CEN Case Rep 2024. Online ahead of print. 10.1007/s13730-024-00859-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakatani S, Morioka F, Uedono H et al. Dapagliflozin administration for 1 year promoted kidney enlargement in patient with ADPKD. CEN Case Rep 2023. Online ahead of print. 10.1007/s13730-023-00840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol: CJASN 2006;1:148–57. 10.2215/CJN.00330705 [DOI] [PubMed] [Google Scholar]
- 15. Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. 10.1161/CIRCULATIONAHA.113.005081 [DOI] [PubMed] [Google Scholar]
- 16. Kim NH, Kim NH. Renoprotective mechanism of sodium-glucose cotransporter 2 inhibitors: focusing on renal hemodynamics. Diabetes Metab J 2022;46:543–51. 10.4093/dmj.2022.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heerspink HJ, Perkins BA, Fitchett DH et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–72. 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 18. Barrett BJ, Foley R, Morgan J et al. Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int 1994;46:1118–23. 10.1038/ki.1994.374 [DOI] [PubMed] [Google Scholar]
- 19. Sinha F, Federlein A, Biesold A et al. Empagliflozin increases kidney weight due to increased cell size in the proximal tubule S3 segment and the collecting duct. Front. Pharmacol 2023;14:1118358. 10.3389/fphar.2023.1118358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vallon V. Glucose transporters in the kidney in health and disease. Pflugers Arch—Eur J Physiol 2020;472:1345–70. 10.1007/s00424-020-02361-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li SR, Gulieva RE, Helms L et al. Glucose absorption drives cystogenesis in a human organoid-on-chip model of polycystic kidney disease. Nat Commun 2022;13:7918. 10.1038/s41467-022-35537-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiaravalli M, Rowe I, Mannella V et al. 2-Deoxy-D-glucose ameliorates PKD progression. JASN 2016;27:1958–69. 10.1681/ASN.2015030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraus A, Peters DJM, Klanke B et al. HIF-1α promotes cyst progression in a mouse model of autosomal dominant polycystic kidney disease. Kidney Int 2018;94:887–99. 10.1016/j.kint.2018.06.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.


