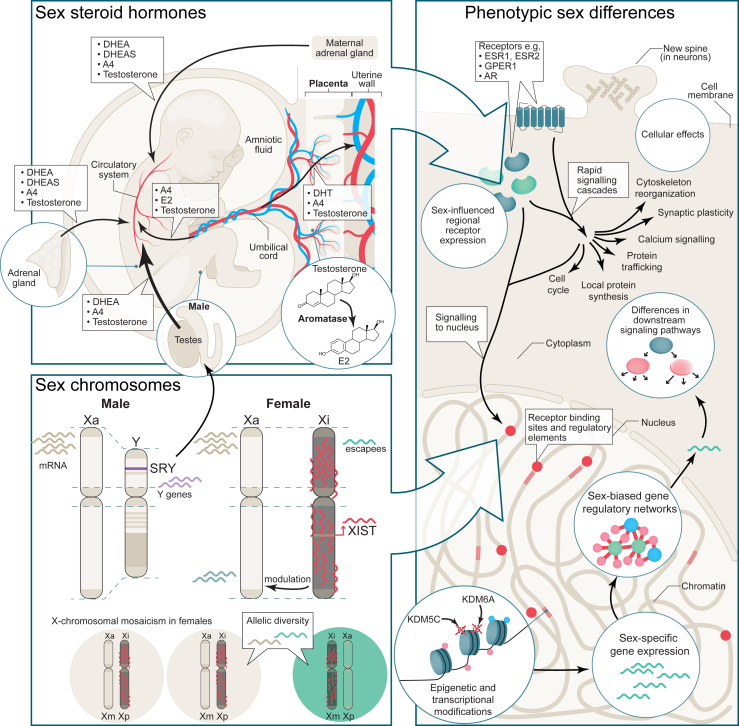
Figure 2.
Sex steroid hormones from various sources (top left) and sex chromosome effects (bottom left) give rise to phenotypic sex differences at the cellular level. Note that although we show the placenta in the context of hormones, the placenta can influence sex differences both through sex steroid hormones or sex chromosome complement. Sex steroid hormones in the fetus can originate from the maternal adrenal gland, the placenta, and from the fetal adrenal gland. After entering the fetal circulatory system, hormones can cross the blood-brain barrier to affect brain development. In males, the testes develop and are a source of androgens. DHEA, DHEAS, A4, E2 are examples of key steroid sex hormones. Aromatase (bottom right) can aromatize testosterone to E2. The sex chromosomes (bottom left) can be a source of gene expression differences. In males, there is 1 active X chromosome and the Y chromosome. The SRY gene on the Y chromosome is responsible for testes development. Expression of other Y genes (purple) that have diverged from their X chromosome homologs in evolution can be a source of sex differences. Females have 1 Xa and 1 Xi. Sex-specific gene expression can originate from genes that are expressed from the Xi (escapees) or from modulation of gene expression on the Xa by the Xi. Finally, X-chromosomal mosaicism in females results in allelic diversity (bottom). The placenta is one example of a tissue where sex chromosome complement has an important role in sex differences. Examples of how various cellular and molecular brain sex differences may arise are shown (right). Exposure to steroid hormones can have rapid cellular effects mediated by rapid signaling cascades or canonical gene expression effects through nuclear signaling. Sex chromosome gene expression effects combined with gene expression in response to steroid hormone signaling can modulate regional receptor expression and steroid signaling pathways, resulting in further sex-specific cellular responses. The combined action of the sex chromosomes and steroid hormones can establish sex-biased gene expression, gene regulatory networks, and epigenetic modifications. One example of epigenetic modifications is demethylation, such as by the KDM5C and KDM6A demethylases, which are discussed. A4, androstenedione; AR, androgen receptor; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; ESR, estrogen receptor; GPER1, G protein–coupled estrogen receptor 1; Xa, active X chromosome; Xi, inactive X chromosome; Xm, X maternal; Xp, X paternal.