Table 1.
Recent studies that conducted affinity chromatography-based target identification of natural products.
| No. | Natural product | Structure | Category | Bioactivity | Target protein | Reference |
|---|---|---|---|---|---|---|
| 1 | Artesunate | 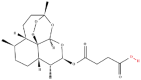 |
Quaternary ammonium salts | Regeneration of pancreatic β cell mass from α cells | Gephyrin | [4] |
| 2 | Arzanol | 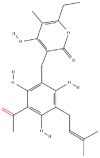 |
Terpenoids | Anti-inflammatory activity | Brain glycogen phosphorylase | [5] |
| 3 | Baicalein | 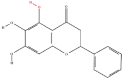 |
Flavonoids | Elimination of liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition | SAR1B guanosine triphosphatase | [6] |
| 4 | 20(S)-Protopanaxadiol |  |
Saponins | Reduction of fatigue | CK-MM | [7] |
| 5 | IJ-5 | 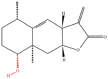 |
Terpenoids | Anti-inflammatory activity | Ubiquitin-conjugating enzyme UbcH5 | [8] |
| 6 | Sappanone A |  |
Ketones | Anti-neuroinflammatory activity | IMPDH2 | [10] |
| 7 | Celastrol | 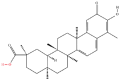 |
Terpenoids | Intervention of the progression of hepatocellular carcinoma | ROCK2 | [11] |
| 8 | Ainsliadimer A | 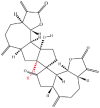 |
Terpenoids | Anticancer and anti-inflammatory activity | IKKβ | [12] |
| 9 | Pateamine A |  |
Alkaloids | Inhibition of eukaryotic translation initiation | Eukaryotic initiation factor 4A | [13] |
| 10 | Adenanthin |  |
Terpenoids | Induction of leukemic cell differentiation | Peroxiredoxins I and II | [14] |
Note: The red-marked portion in the structure refers to the labeling binding site.
