Abstract
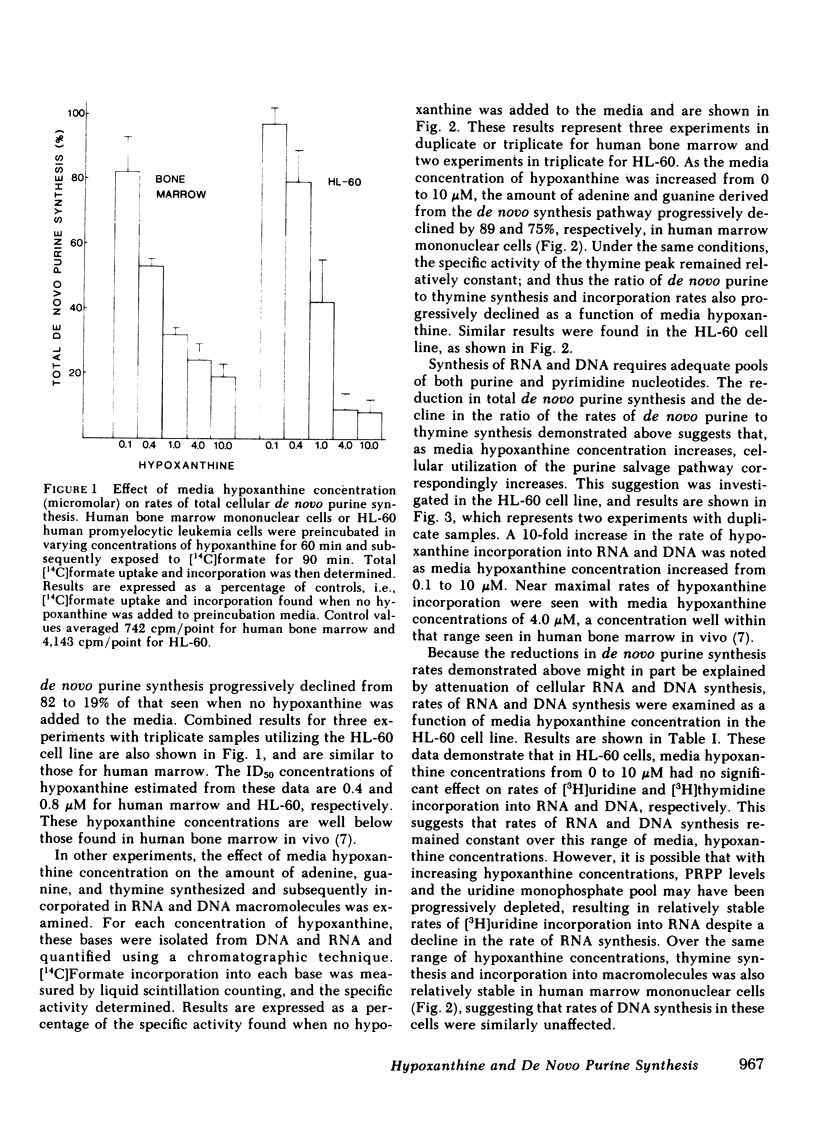
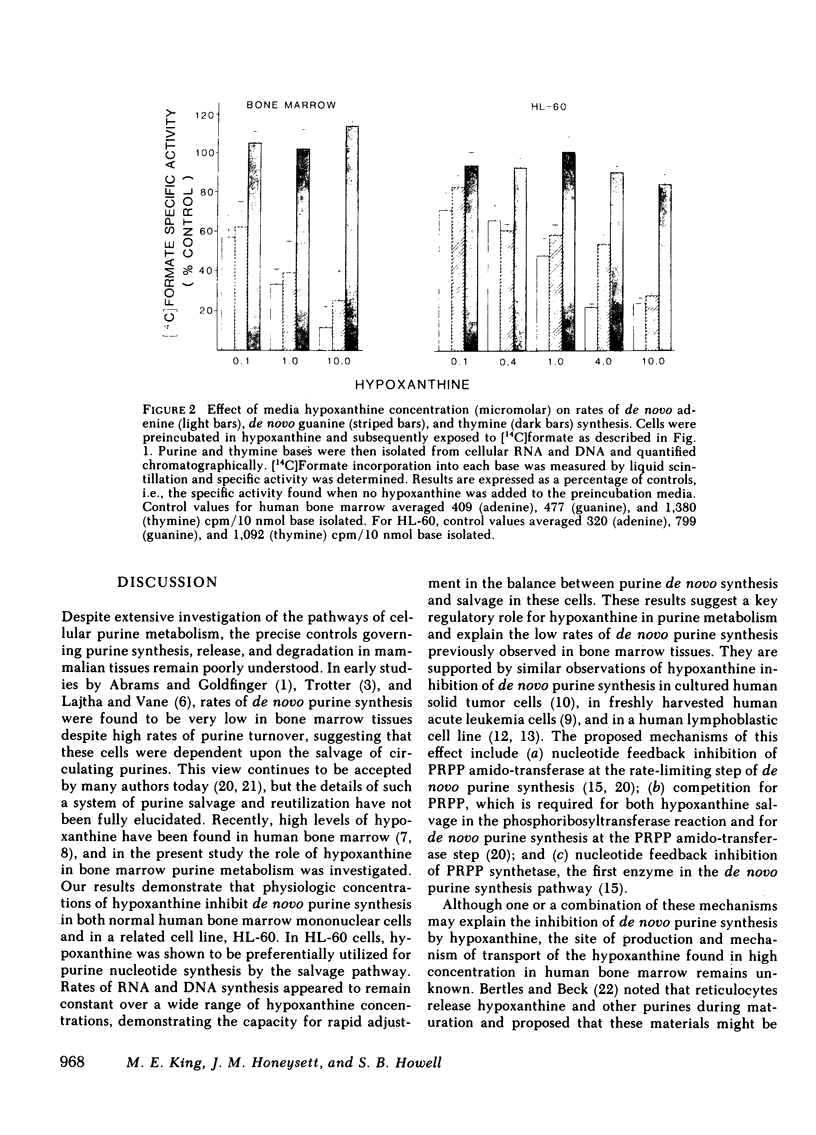
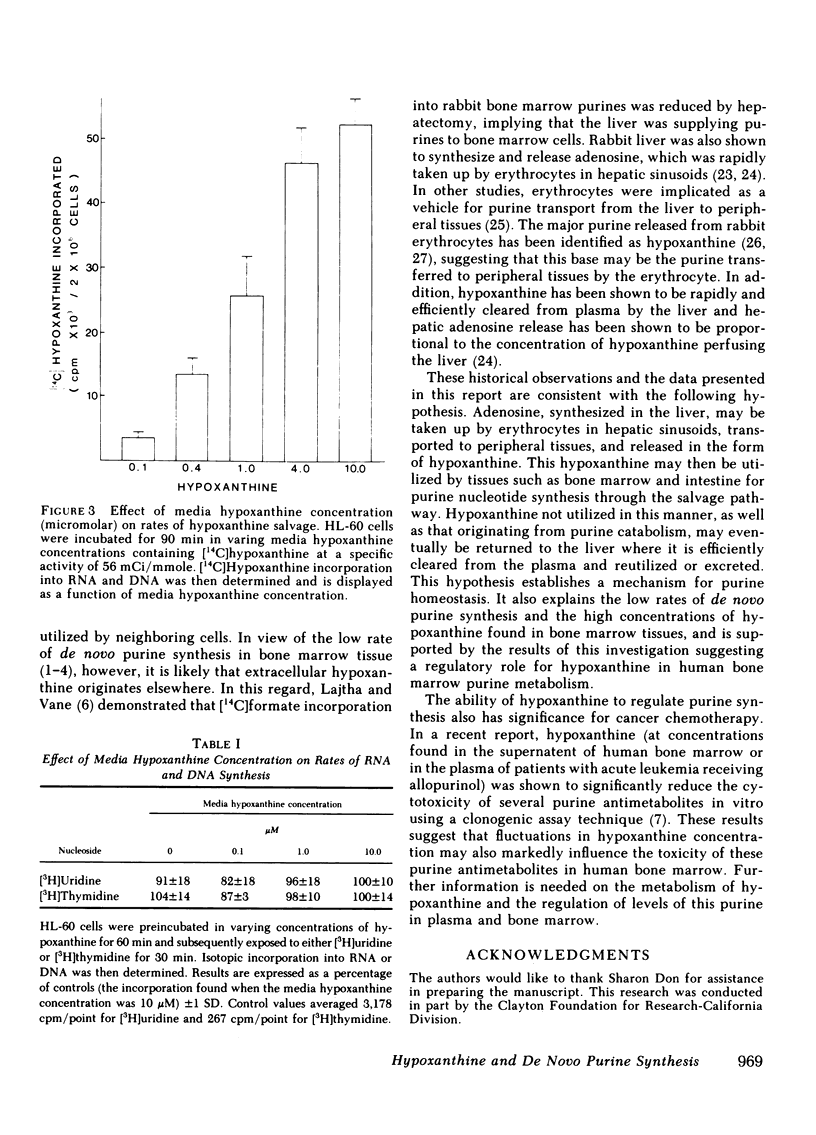
In previous studies from this laboratory, human bone marrow hypoxanthine concentrations were found to average 7.1 microM, three times higher than plasma hypoxanthine concentrations measured simultaneously. To assess the significance of this finding, the relationship between hypoxanthine concentration and the rate of purine nucleotide synthesis by the de novo pathway was studied in normal human bone marrow mononuclear cells and in the human promyelocytic cell line, HL-60, in vitro. Utilizing a [14C]formate incorporation technique, rates of total cellular de novo purine synthesis as well as rates of de novo adenine, de novo guanine, and thymine synthesis and incorporation into RNA and DNA were measured as a function of hypoxanthine concentration. In normal human marrow cells, the rate of total de novo purine synthesis declined by 81%, while the rate of de novo adenine and de novo guanine synthesis and incorporation into macromolecules declined by 89 and 75%, respectively, when media hypoxanthine was increased from 0 to 10 microM. Similar results were seen in the HL-60 cell line. In contrast, rates of thymine synthesis and incorporation into DNA as well as overall rates of RNA and DNA synthesis did not change with varying media hypoxanthine concentrations. In addition, hypoxanthine salvage and incorporation into RNA and DNA was shown to progressively increase with increasing media hypoxanthine concentrations. These results indicate that physiologic concentrations of hypoxanthine are sufficient to regulate the rate of de novo purine synthesis in human bone marrow in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS R., GOLDINGER J. M. Formation of nucleic acid purines from hypoxanthine and formate in bone marrow slices. Arch Biochem Biophys. 1952 Feb;35(2):243–248. doi: 10.1016/s0003-9861(52)80003-7. [DOI] [PubMed] [Google Scholar]
- ABRAMS R., GOLDINGER J. M. Utilization of purines for nucleic acid synthesis in bone marrow slices. Arch Biochem. 1951 Feb;30(2):261–268. [PubMed] [Google Scholar]
- BROCKMAN R. W., CHUMLEY S. INHIBITION OF FORMYLGLYCINAMIDE RIBONUCLEOTIDE SYNTHESIS IN NEOPLASTIC CELLS BY PURINES AND ANALOGS. Biochim Biophys Acta. 1965 Mar 15;95:365–379. doi: 10.1016/0005-2787(65)90183-8. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO K. Y. ON THE MECHANISM OF FEEDBACK INHIBITION OF PURINE BIOSYNTHESIS DE NOVO IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jul;240:3104–3109. [PubMed] [Google Scholar]
- HENDERSON J. F., LE PAGE G. A. Transport of adenine-8-C14 among mouse tissues by blood cells. J Biol Chem. 1959 Dec;234:3219–3223. [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine biosynthesis in human lymphoblasts. Coordinate control of proximal (rate-determining) steps and the inosinic acid branch point. J Biol Chem. 1976 Dec 10;251(23):7348–7354. [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine synthesis in human lymphoblasts. Similar rates of de novo synthesis during growth by normal cells and mutants deficient in hypoxanthine-guanine phosphoribosyltransferase activity. J Biol Chem. 1977 Sep 10;252(17):6002–6010. [PubMed] [Google Scholar]
- Hershko A., Razin A., Shoshani T., Mager J. Turnover of purine nucleotides in rabbit erythrocytes. II. Studies in vitro. Biochim Biophys Acta. 1967 Nov 21;149(1):59–73. doi: 10.1016/0005-2787(67)90691-0. [DOI] [PubMed] [Google Scholar]
- Itakura M., Sabina R. L., Heald P. W., Holmes E. W. Basis for the control of purine biosynthesis by purine ribonucleotides. J Clin Invest. 1981 Apr;67(4):994–1002. doi: 10.1172/JCI110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- LAJTHA L. G., VANE J. R. Dependence of bone marrow cells on the liver for purine supply. Nature. 1958 Jul 19;182(4629):191–192. doi: 10.1038/182191a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. H., Lowy B. A. The formation of adenosine in rabbit liver and its possible role as a direct precursor of erythrocyte adenine nucleotides. J Biol Chem. 1974 Feb 10;249(3):959–966. [PubMed] [Google Scholar]
- Mackinnon A. M., Deller D. J. Purine nucleotide biosynthesis in gastrointestinal mucosa. Biochim Biophys Acta. 1973 Aug 10;319(1):1–4. doi: 10.1016/0005-2787(73)90034-8. [DOI] [PubMed] [Google Scholar]
- Mager J., Hershko A., Zeitlin-Beck R., Shoshani T., Razin A. Turnover of purine nucleotides in rabbit erythrocytes. I. Studies in vivo. Biochim Biophys Acta. 1967 Nov 21;149(1):50–58. doi: 10.1016/0005-2787(67)90690-9. [DOI] [PubMed] [Google Scholar]
- Murray A. W. The biological significance of purine salvage. Annu Rev Biochem. 1971;40:811–826. doi: 10.1146/annurev.bi.40.070171.004115. [DOI] [PubMed] [Google Scholar]
- Pritchard J. B., O'Connor N., Oliver J. M., Berlin R. D. Uptake and supply of purine compounds by the liver. Am J Physiol. 1975 Oct;229(4):967–972. doi: 10.1152/ajplegacy.1975.229.4.967. [DOI] [PubMed] [Google Scholar]
- SCOTT J. L. Human leukocyte metabolism in vitro. I. Incorporation of adenine-8-C-14 and formate-C-14 into the nucleic acids of leukemic leukocytes. J Clin Invest. 1962 Jan;41:67–79. doi: 10.1172/JCI104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOTTER J. R., BEST A. N. The metabolism of formate-C14 by rabbit bone marrow in vitro. Arch Biochem Biophys. 1955 Feb;54(2):318–329. doi: 10.1016/0003-9861(55)90043-6. [DOI] [PubMed] [Google Scholar]
- Thompson L. F., Willis R. C., Stoop J. W., Seegmiller J. E. Purine metabolism in cultured human fibroblasts derived from patients deficient in hypoxanthine phosphoribosyltransferase, purine nucleoside phosphorylase, or adenosine deaminase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3722–3726. doi: 10.1073/pnas.75.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Robins R. K., Seegmiller J. E. An in vivo and in vitro evaluation of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine: an inhibitor of human lymphoblast purine nucleoside phosphorylase. Mol Pharmacol. 1980 Sep;18(2):287–295. [PubMed] [Google Scholar]