Figure 7. Summary of key findings from current study: EPA‐mediated endothelial protection during inflammation.
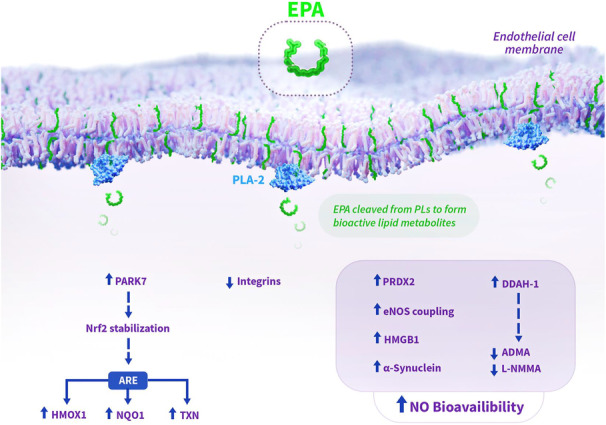
EPA facilitates improved NO bioavailability and endothelial cell protection through various mechanisms elucidated in the current study. EPA is known to concentrate in phospholipids in the cell membrane, where it can be liberated by PLA‐2 to activate various intracellular actions. By improving eNOS coupling efficiency, attenuating oxidative stress, and modulating endogenous inhibitors (L‐NMMA, ADMA), EPA treatment abates endothelial dysfunction. Additionally, EPA increases expression of PARK7, which stabilizes Nrf2 to increase expression of genes within ARE in DNA, including HMOX‐1, TXN, and NQO1. Increased expression of α‐synuclein with EPA may also contribute to the improved NO bioavailability, as recombinant α‐synuclein has been shown to improve NO release. EPA‐mediated increased levels of HMGB1 may represent a novel mechanism for improved NO bioavailability, as intracellular HMGB1 has been shown to be required for normal vasomotor function and NO release. Solid arrows indicate observed changes with EPA treatment in the current study, while dashed arrows represent hypothesized effects based on changes in protein expression with EPA treatment. ADMA indicates asymmetric dimethylarginine; ARE, antioxidant response element; DDAH‐1, dimethylarginine dimethylaminohydrolase‐1; eNOS, endothelial nitric oxide synthase; EPA, eicosapentaenoic acid; HMGB1, high mobility group box protein 1; HMOX1, heme oxygenase‐1; IL‐6, interleukin‐6; L‐NMMA, N(G)‐monomethyl L‐arginine; NO, nitric oxide; NQO1, NAD(P)H quinone oxidoreductase‐1; Nrf2, nuclear factor erythroid 2‐related factor; PARK7, Parkinson's Disease protein 7; PLs, phospholipids; PLA‐2, phospholipase A2; PRDX2, peroxiredoxin‐2; and TXN, thioredoxin.
