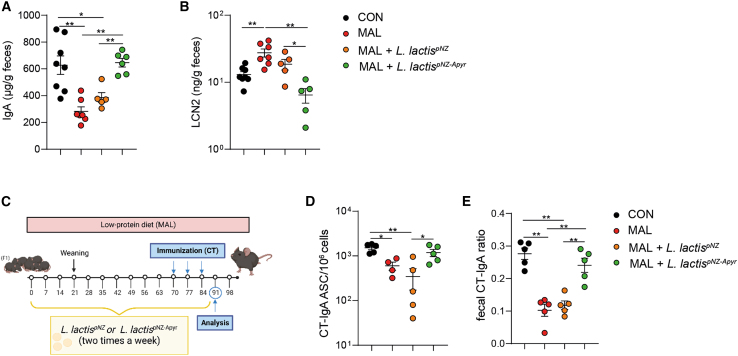
Figure 7.
Lactococcus lactispNZ-Apyr administration ensures a proficient IgA production in adulthood and improves IgA response to oral CT immunization in MAL mice
(A) Quantification of total fecal IgA by ELISA in 10-week-old CON mice, untreated MAL mice, and MAL mice treated with L. lactispNZ or L. lactispNZ-Apyr.
(B) Fecal LCN-2 concentration (ng/g feces), (Mann-Whitney U test). Data points represent individual mice, and statistics are displayed as mean ± SEM.
(C) Experimental design. After weaning, untreated MAL mice or MAL mice treated with L. lactispNZ or L. lactispNZ-Apyr were maintained in low-protein diet and were gavaged with L. LactispNZ or L. lactispNZ-Apyr twice a week for the entire duration of the experiment. At 10 weeks of age, CON mice and untreated MAL mice or MAL mice treated with L. lactispNZ or L. lactispNZ-Apyr were orally immunized with CT at day 70, 77, and 84. At day 91, mice were analyzed.
(D) CT-specific IgA-secreting plasma cells measured by ELISPOT assay in the lamina propria of the small intestine from CON mice, untreated MAL mice, and MAL mice treated with L. lactispNZ or L. lactispNZ-Apyr.
(E) Quantification of CT-specific IgA to total IgA ratio in feces of CON mice, untreated MAL mice, and MAL mice treated with L. lactispNZ or L. lactispNZ-Apyr (Mann-Whitney U test). Data points represent individual mice, and statistics are displayed as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01.