Abstract
Background
Women with thoracic aortic aneurysms within the arch or descending thoracic aorta have poorer survival than men. Sex differences in relative thoracic aortic aneurysm size may account for some of the discrepancy. The aim of this study was to explore whether basing clinical management on aneurysm size index (maximum aneurysm diameter/body surface area) rather than aneurysm size can restore equality of survival by sex.
Methods
The Effective Treatments for Thoracic Aortic Aneurysms (ETTAA; ISRCTN04044627) study was a prospective, observational cohort study. Adults referred to National Health Service hospitals in England with new/existing arch or descending thoracic aorta aneurysms greater than or equal to 4 cm in diameter were followed from March 2014 to March 2022. Baseline characteristics and survival to intervention and overall were compared for men and women. Survival models were used to assess the association between all-cause survival and sex, with and without adjustment for aneurysm diameter or aneurysm size index.
Results
A total of 886 thoracic aortic aneurysm patients were recruited: 321 (36.2%) women and 565 (63.8%) men. The mean(s.d.) aneurysm diameter was the same for women and men (5.7(1.1) versus 5.7(1.2) cm respectively; P = 0.751), but the mean(s.d.) aneurysm size index was greater for women than for men (3.32(0.80) versus 2.83(0.63) respectively; P < 0.001). Women had significantly worse survival without intervention: 110 (34.3%) women and 135 (23.9%) men (log rank test, P < 0.001). All-cause mortality remained greater for women after adjustment for diameter (HR 1.65 (95% c.i. 1.35 to 2.02); P < 0.001), but was attenuated after adjustment for aneurysm size index (HR 1.11 (95% c.i. 0.89 to 1.38); P = 0.359). Similar results were found for all follow-up, with or without intervention, and findings were consistent for descending thoracic aorta aneurysms alone.
Conclusion
Guidelines for referral to specialist services should consider including aneurysm size index rather than diameter to reduce inequity due to patient sex.
This study examined the effect of sex on overall and aneurysm-related survival and whether stratification by aneurysm size index (maximum aneurysm diameter/body surface area) can restore equality of survival by sex. It identified that all-cause mortality remained greater for women after adjustment for diameter (HR 1.65 (95% c.i. 1.35 to 2.02); P < 0.001), but was attenuated after adjustment for aneurysm size index (HR 1.11 (95% c.i. 0.89 to 1.38); P = 0.359), with similar observations for aneurysm-related mortality and those without or after intervention. Guidelines for referral to specialist services should be based on aneurysm size index rather than diameter to reduce inequity due to patient sex.
Introduction
Thoracic aortic aneurysms (TAAs) are often asymptomatic, but can cause considerable mortality and morbidity when progression to aortic rupture or dissection occurs1. The estimated annual incidence is 5.3 (95% c.i. 3.0 to 8.3) TAAs per 100 000, with a prevalence of 0.16% (95% c.i. 0.12% to 0.20%)2. These figures are probably underestimated, due to misattribution of sudden death from the rupture of asymptomatic TAAs to other causes3. When diagnosed early, small TAAs can be monitored until the risk of rupture approaches the risk of intervention, which can be performed using endovascular stent grafting (ESG) or open surgical repair (OSR)3.
Women are less likely to have TAAs identified than men, but are up to three times more likely to have TAAs that dissect or rupture at smaller sizes4,5. Degenerative TAAs in women also demonstrate faster growth rates6,7. Sex differences in relative TAA size may account for some of the discrepancies observed and it has been suggested that use of indexing may improve the prediction of dissection risk8,9. Although international guidelines recognize that the thoracic aorta is smaller for women, only the European Society for Vascular Surgery suggests that the threshold for surgery could be reduced and all highlight that the quality of the available evidence remains low10–12. Further research is encouraged, including sex-based analysis of large national and international registries of TAAs13.
The Effective Treatments for Thoracic Aortic Aneurysms (ETTAA) study was a prospective, observational study of patients presenting with TAAs in the arch and descending thoracic aorta in England3,14. Although the primary aim was to compare clinical and quality of life outcomes for different management protocols, it also found that women had poorer survival outcomes, despite similar-sized TAAs. This data set provides an opportunity to assess the differences in presentation, treatment, and outcomes for women compared with men and to assess the need for changes in guidelines to address their poorer survival. The aims of this study were to: describe differences between men and women referred for investigation and treatment in the ETTAA study; explore effects of sex on overall and aneurysm-related (AR) survival; assess the ability of stratification by aneurysm size index (ASI) to restore equality of survival by sex; and explore patient and aneurysm characteristics that impact on survival, in addition to sex and aneurysm size.
Methods
Declaration of Helsinki
The authors confirm that the ETTAA study complies with the Declaration of Helsinki, that the West Midlands—South Birmingham Research Ethics Committee approved the research protocol, and that informed consent was obtained from all participants.
Original Effective Treatments for Thoracic Aortic Aneurysms study
Details of the ETTAA study (ISRCTN04044627) have been published and are further described in the Supplementary Methods3,14. The study recruited adults (greater than or equal to 18 years) presenting to UK National Health Service (NHS) hospitals with new or existing TAAs greater than or equal to 4 cm in diameter in the arch, descending, or thoracoabdominal aorta. The main exclusion criteria were acute dissection ± malperfusion syndromes and previous intervention for the same aneurysm. Within the ETTAA study, patient sex was recorded as a dichotomous variable (male/female).
Baseline variables included patient characteristics (age, height, BMI, need for formal/informal care, and smoking), aneurysm characteristics (maximum aneurysm diameter and location in the aorta), co-morbidities (connective tissue disorders, diabetes, extra-cardiac arteriopathy, treated coronary heart disease, heart valve disease, hypertension, chronic obstructive pulmonary disease, and New York Heart Association (NYHA) classification of breathlessness), and cardiac medication (Table 1 and Tables S1–S4). Extra-cardiac arteriopathy was defined as the presence of any of the following: claudication; carotid occlusion or greater than 50% stenosis; or previous or planned intervention on the abdominal aorta, limb arteries, or carotids. Treatment for coronary artery disease included coronary artery bypass grafting, percutaneous coronary intervention, and medication. Medical treatment was heterogeneous and often involved multiple drugs. Collection of serum creatinine and haemoglobin levels was not mandated in the ETTAA study and levels were unavailable for more than half the patients and so were not analysed in the present study. ASI was calculated as maximum aneurysm diameter (cm) divided by body surface area (m2) using the formula of Du Bois and Du Bois8,15.
Table 1.
Patient characteristics and co-morbidities at baseline by sex
| Variable | Female (n = 321) | Male (n = 565) | P |
|---|---|---|---|
| Age (years), mean(s.d.)—missing 0 | 72.8 (9.9) | 69.7 (11.2) | <0.001 |
| Height (cm), mean(s.d.)—missing 12 women, 23 men | 161.6 (7.7) | 176.3 (8.4) | <0.001 |
| BMI (kg/m2), mean(s.d.)—missing 16 women, 26 men | 26.7 (5.3) | 27.7 (4.5) | 0.003 |
| Formal/informal care—missing 5 women, 2 men | 49 (15.5) | 54 (9.6) | 0.009 |
| Smoking—missing 1 women, 6 men | |||
| Current smoker | 53 (16.6) | 60 (10.7) | 0.045 |
| Ex-smoker | 176 (55.0) | 327 (58.5) | |
| Never smoked | 91 (28.4) | 172 (30.8) | |
| Connective tissue disorder—missing 0 | 20 (6.2) | 35 (6.2) | 0.983 |
| Extra-cardiac arteriopathy—missing 4 women, 11 men | 33 (10.4) | 100 (18.0) | 0.003 |
| Type I/II diabetes—missing 0 women, 3 men | 22 (6.8) | 61 (10.8) | 0.050 |
| Treated coronary artery disease—missing 7 women, 10 men | 47 (15.0) | 121 (21.8) | 0.014 |
| Hypertension—missing 1 women, 1 men | 271 (84.7) | 504 (89.4) | 0.042 |
| Heart valve disease—missing 6 women, 9 men | 74 (23.5) | 91 (16.4) | 0.010 |
| Chronic obstructive pulmonary disease—missing 4 women, 1 men | 84 (26.5) | 79 (14.0) | <0.001 |
| NYHA classification of breathlessness—missing 9 women; 27 men | |||
| I | 113 (36.2) | 246 (45.7) | 0.001 (I/II versus III/IV) |
| II | 117 (37.5) | 198 (36.8) | |
| III | 65 (20.8) | 85 (15.8) | |
| IV | 17 (5.5) | 9 (1.7) | |
| EQ-5D-5L index, mean(s.d.)—missing 1 women, 6 men | 0.68 (0.3) | 0.74 (0.2) | 0.001 |
Values are n (%) unless otherwise indicated. P values are for comparisons excluding missing data. NYHA, New York Heart Association; EQ-5D-5L, EuroQoL, five dimensions, five levels.
The primary outcomes were all-cause and AR mortality. Deaths were classified as AR or non-AR by local investigators when they occurred during the ETTAA study interval. Deaths recorded in Hospital Episode Statistics (HES) after this interval were classified as AR when ICD-10 codes included I710, I711, I712, I715, I716, I718, or I719. Two time frames were considered: first, to any surgical intervention (ESG or OSR); and second to the end of the study (with or without surgical intervention).
Extended follow-up for patients in the Effective Treatments for Thoracic Aortic Aneurysms study
Follow-up for the ETTAA study was completed on 30 June 2019. The ETTAA study has Health Research Authority approval (IRAS 140264) and Research Ethics Committee approval (13/WM/0507) and these remain active to allow for collection and analysis of NHS Digital data. Linking ETTAA study data to nationwide electronic health records via the UK National HES, follow-up for surgical intervention and survival was extended to March 202216.
Statistical methods
To assess the imbalance in baseline variables on a common scale, standardized mean differences (SMDs) were calculated as the mean differences between men and women, divided by the standard deviations of the pooled data. A value of one (or −1) meant that the mean difference between the sexes was one standard deviation. A difference of ±0.1 in the SMD was used as an informal threshold for imbalance17.
To explore survival for women and men with similar aneurysm size, patients were split into three equal-sized strata according to diameter and survival was summarized using stratified Kaplan–Meier plots. Patients were then split into three equal-sized strata according to ASI and the analysis was repeated.
Cox proportional hazard models were used to assess whether all-cause survival was associated with sex, with and without adjustment for aneurysm size or ASI, in the two time frames. To analyse all-cause mortality up to surgical intervention, competing risk models were used, with death as the event of interest and intervention (ESG or OSR) the competing risk18.
Associations between AR deaths and sex were analysed using competing risk models, with deaths from causes other than AR events (and intervention for the first time frame) treated as competing risks.
To explore variables that impacted on survival, in addition to sex and aneurysm size, patient characteristics and co-morbidities were added to the model based on improvement in fit according to the Akaike Information Criterion (AIC). Interactions between time and each covariate were used to assess non-proportional hazards.
The primary analysis used only patients with complete baseline data; 109 (12.3%) patients had at least one missing baseline variable. In the sensitivity analysis, the results for the main analyses were checked using multiply (×15) imputed data for missing baseline variables (see the Supplementary material)19. To assess whether patient height was better than body surface area in explaining sex-specific differences in survival, the main analyses were repeated using maximum aneurysm diameter divided by height (aneurysm height index (AHI)). The main analyses were repeated using patients with aneurysms with maximum aneurysm diameter in the descending thoracic aorta.
Results
Differences between sexes at presentation
Between March 2014 and June 2018, 886 TAA patients were recruited: 321 (36.2%) women and 565 (63.8%) men. Women were a mean of 3.1 (95% c.i. (1.6 to 4.6) years older than men (P < 0.001) and more likely to require formal/informal care (Table 1). Women were shorter than men (mean difference −14.7 cm (95% c.i. −15.9 to −13.6); P < 0.001) and had a lower BMI (mean difference −1.0 kg/m2 (95% c.i. −1.7 to −0.4); P = 0.003).
Table 1 shows important differences between men and women at presentation. Women were significantly more likely to have concomitant heart valve disease, chronic obstructive pulmonary disease, and NYHA classification of breathlessness III/IV; in addition, they were more likely to be current smokers. Men were significantly more likely to have concomitant extra-cardiac arteriopathy, type I/II diabetes, and treatment for coronary artery disease. Although most patients had hypertension, it was slightly more prevalent in men. Men were significantly more likely to be treated for both hypertension (87.1% versus 81.3% for men and women respectively; P = 0.021) and hypercholesterolaemia (61.6% versus 51.1% for men and women respectively; P = 0.002) (Table S1).
The EuroQoL, five dimensions, five levels (EQ-5D-5L) questionnaire was completed at baseline by 879 patients20. Mean health-related quality of life was significantly lower for women (difference in EQ-5D-5L index −0.06 (95% c.i. −0.01 to −0.02); P = 0.001) (Table S3).
There were minimal differences between women and men regarding the location where the maximum aneurysm diameter occurred (in the descending thoracic aorta/thoracoabdominal aorta for 81.6% for women versus 83.5% for men; P = 0.466) and the maximum aneurysm diameter (mean(s.d.) 5.7(1.1) cm for women and 5.7(1.2) cm for men; P = 0.751) (Table S4). Women had a significantly higher ASI than men (mean(s.d.) 3.32(0.80) versus 2.83(0.63) cm/m2 respectively; P < 0.001).
Figure S1 shows SMDs between men and women for important baseline variables. Using this common scale, women and men were well matched for location and maximum diameter of the aneurysm and prevalence of connective tissue disorders, with minor imbalance for age and cardiac/respiratory symptoms and co-morbidities. However, there was marked imbalance between the sexes regarding ASI.
Differences in patient management
During follow-up to March 2022, 60 (18.7%) women and 120 (21.2%) men had ESG as a first procedure, whereas 71 (22.1%) women and 120 (21.2%) men had OSR as a first procedure. The remaining 190 (59.2%) women and 325 (57.5%) men were in the CM (48 (15.0%) women and 64 (11.3%) men) or WW (142 (44.2%) women and 261 (46.2%) men) groups at the end of follow-up or died without surgical intervention. There were no differences between the sexes regarding the management group at the end of the study (chi-squared test, P = 0.258) and the time to treatment (log rank test, P = 0.969).
Survival during the first time frame (up to intervention) by sex
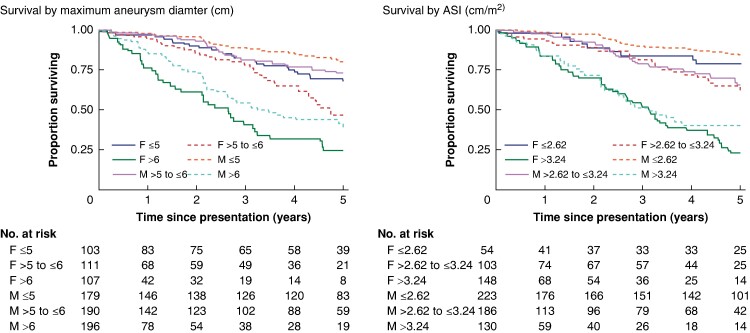
During extended follow-up, 110 (34.3%) women and 135 (23.9%) men died without intervention; 48 of 110 (43.6%) deaths among women were categorized as AR, compared with 48 of 135 (35.6%) deaths among men. There was a significant difference between the sexes regarding overall survival without surgical intervention (log rank test, P < 0.001) (Fig. S2). Stratifying into three equally sized groups by maximum diameter or ASI, 107 (33.3%) women were in the largest third for maximum diameter, but 148 (48.5%) women were in the largest third for ASI (Table S5). Figure 1 (left panel) shows that women with the smallest aneurysms (less than or equal to 5 cm) had similar survival to men with aneurysms greater than 5 to less than or equal to 6 cm (middle third) and that survival for men with large aneurysms (greater than 6 cm) was between that for women with medium (greater than 5 to less than or equal to 6 cm) and large (greater than 6 cm) aneurysms. In contrast, when stratifying patients according to ASI, survival patterns for the two sexes were broadly aligned (Fig. 1, right panel). For example, survival probability at 2 years for women and men with medium-sized aneurysms was 85.1% (95% c.i. 75.1% to 91.3%) and 91.2% (95% c.i. 85.3% to 94.8%) respectively and the corresponding survival probabilities for a medium-sized ASI were 89.6% (95% c.i. 80.8% to 94.4%) and 89.6% (95% c.i. 82.6% to 93.9%) respectively.
Fig. 1.
All-cause survival without surgical intervention stratified by sex and maximum aneurysm diameter (left panel) and by sex and aneurysm size index (right panel)
ASI, aneurysm size index.
The patterns for AR survival were similar, although the smaller number of events, especially for small aneurysms, meant that the improved alignment was mostly evident for people with large aneurysms, among whom there were more deaths (Figs S3, S4).
Table 2 shows that for both all-cause and AR mortality, the significant effect of sex on survival without intervention was slightly inflated when adjusted for maximum aneurysm diameter, but was attenuated and non-significant when adjusted for ASI.
Table 2.
HRs (or sub-distribution HRs) for all-cause and aneurysm-related mortality for sex alone (unadjusted) and for sex adjusted for aneurysm diameter or aneurysm size index
| Outcome model | HR (95% c.i.) for sex | P | HR (95% c.i.) for sex adjusted for aneurysm diameter | P | HR (95% c.i.) for sex adjusted for aneurysm size index | P |
|---|---|---|---|---|---|---|
| Without intervention—all-cause mortality (SHR)* | 1.54 (1.20,1.98) | 0.001 | 1.56 (1.21,2.01) | 0.001 | 1.24 (0.94,1.65) | 0.132 |
| Without intervention—aneurysm-related mortality (SHR)* | 1.82 (1.22,2.72) | 0.003 | 1.89 (1.26,2.84) | 0.002 | 1.26 (0.81,1.95) | 0.314 |
| With/without intervention—all-cause mortality† | 1.56 (1.28,1.91) | <0.001 | 1.65 (1.35,2.02) | <0.001 | 1.11 (0.89,1.38) | 0.359 |
| With/without intervention—aneurysm-related mortality (SHR)† | 2.13 (1.57,2.90) | <0.001 | 2.35 (1.71,3.23) | <0.001 | 1.47 (1.05,2.07) | 0.026 |
*Includes follow-up until intervention or end of study. †Includes all follow-up with or without surgical intervention. SHR, sub-distribution HR.
Survival with or without surgery (all follow-up) by sex
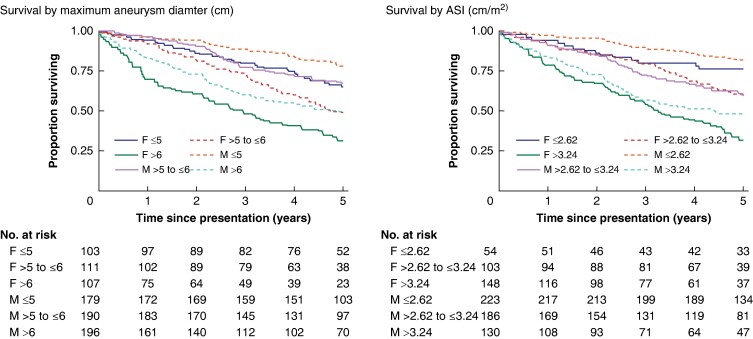
An additional 64 (48.9%) women and 96 (40.0%) men died after intervention (see Fig. S5 for post-surgery outcomes). Overall, 172 (53.6%) women and 224 (39.7%) men died by the end of extended follow-up, with Kaplan–Meier all-cause mortality curves showing non-overlapping confidence intervals for women and men (log rank test, P < 0.001) (Fig. S6). Figure 2 again shows better alignment between the two sexes when survival was stratified for ASI than when stratified by maximum aneurysm diameter.
Fig. 2.
Overall all-cause survival stratified by sex and maximum aneurysm diameter (left panel) and by sex and aneurysm size index (right panel)
ASI, aneurysm size index.
Differences between the sexes were observed for AR survival, although the smaller number of events and the intervening surgery for some patients meant the improved alignment was less clear (Figs S7, S8).
Table 2 shows that the effect of sex on all-cause and AR mortality was slightly increased when adjusted for maximum diameter, but was attenuated when adjusted for ASI.
Adjustment for additional confounders
Table 3 shows additional variables that had an important effect on survival outcomes after adjusting for sex and ASI. In most cases, older age showed the next strongest relationship, with estimated HRs between 1.03 and 1.07 per year increase in age (1.34 to 1.97 per decade). For all-cause death, both age and respiratory dysfunction (increasing NYHA classification of breathlessness and chronic obstructive pulmonary disease) were consistently significant across analyses. Patients reporting use of formal/informal care also had a 53% increased hazard of all-cause mortality without intervention. These associations suggest increased risk due to frailty (Table 3 and Table S6).
Table 3.
HRs (or sub-distribution HRs) with 95% confidence intervals for outcomes for additional variables adjusted for sex and aneurysm size index
| Variable | Outcome model | |||
|---|---|---|---|---|
| Without intervention—all-cause mortality (SHR)* | Without intervention—aneurysm-related mortality (SHR)* | With/without intervention—all cause mortality† | With/without intervention—aneurysm-related mortality (SHR)† | |
| Female sex | 1.10 (0.82,1.47) | 1.30 (0.82,2.05) | 0.94 (0.75,1.19) | 1.47 (1.05,2.07) |
| Aneurysm size index (per cm/m2) | 1.43 (1.20,1.72) | 1.92 (1.50,2.46) | 1.90 (1.65,2.18) | 2.17 (1.80,2.61) |
| Age (per year) | 1.07 (1.05,1.10) | 1.03 (1.00,1.06) | 1.05 (1.04,1.06) | – |
| NYHA classification of breathlessness (per class) | 1.32 (1.11,1.56) | – | 1.29 (1.13,1.47) | – |
| Chronic obstructive pulmonary disease | 1.47 (1.06,2.04) | – | 1.54 (1.19,1.98) | – |
| Formal/informal care | 1.53 (1.08,2.17) | – | – | – |
All models include sex and aneurysm size index; other variables included if appropriate (see the Methods section). *Includes follow-up until intervention or end of study. †Includes all follow-up with or without surgical intervention. SHR, sub-distribution HR; NYHA, New York Heart Association.
Sensitivity analysis
Using multiple imputation for missing data changed estimates of effects slightly, but conclusions remained consistent (Tables S7–S9). Repeating the main analysis using AHI rather than ASI to adjust maximum diameter showed similar patterns, but was less effective in aligning survival for women and men (Tables S10, S11).
Repeating the analysis using the 734 patients (262 women and 472 men) with descending thoracic aorta aneurysms, there was no change in the overall conclusions. The biggest changes in results from analysis of the full data set occurred when there were fewer deaths (for example for AR death) and when there were multiple causes of death (for example post-intervention when deaths were also related to intervention, age, and co-morbidities) (Fig. S5). The smaller number of deaths when restricting to AR causes meant that the estimates were less precise (Tables S12–S14).
Discussion
Analysis of this large, multicentre, prospective cohort demonstrates important differences in age, co-morbidities, and quality of life between women and men at the point of referral for investigation of TTAs. Although maximum aneurysm diameter, aneurysm location, and type and timing of intervention are similar, there was a marked and highly significant difference in ASI between men and women. Adjustment for ASI, as a marker of size at baseline relative to patient’s size, largely accounts for the difference in survival outcomes, both before intervention is carried out and for the duration of follow-up with/without intervention. Adjustment for maximum aneurysm diameter does not attenuate the increased risk for women, suggesting that use of diameter thresholds in current guidelines may be insufficient for risk stratification10,11,21. Rather, use of ASI may be a better basis for recommendations around referral for investigation and subsequent intervention. ASI has been criticized due to changing weight over time, with adjustment for height and volumetry proposed22. Adjustment for patient height is also effective in aligning survival outcomes for the two sexes, albeit less so in the ETTAA study. Further to ASI, age and respiratory co-morbidity, as well as frailty (reflected by need for care and low BMI), should play a part in clinical decision-making. Irrespective of sex, there is some evidence that the effect of size decreases over time and the effect of age increases over time, which reflects increasing risk due to multiple age-related co-morbidities in this cohort. The maximum aneurysm diameter occurred in the descending thoracic aorta for over 80% of patients, meaning that the results for this subgroup are consistent with those for the full data set.
The Yale group reports a protective effect for men in a large single-centre study of men with aortic disease5 and advocates the use of ASI for risk stratification8. Inferior outcomes for women are also reported throughout the spectrum of cardiovascular disease23,24. The causes for this discrepancy, including for TAAs, are likely to be multifactorial, including: delayed diagnosis; differences in risk factors, aetiology, and disease progression; under-representation in the research guiding treatment; and subsequent suboptimal management of cardiovascular risk25–29. The International Registry of Acute Aortic Dissection (IRAD) reports delayed diagnosis for women presenting with aortic dissection, attributed to a lower likelihood of presentation with ‘typical’ chest pain symptoms30. Hereditable causes of aortopathies have different aortic risk profiles for men and women31,32. For women with a degenerative TAA under surveillance, enhanced extracellular matrix degradation, adverse arterial haemodynamics, and hypertension have been identified as contributors to faster growth6,7,33–35. The Canadian Thoracic Aortic Collaborative has identified female sex as an independent risk factor for perioperative death, stroke, and complications after thoracic aortic repair, despite use of less complex procedures36, and increased risk of 30-day and 1-year mortality for women was also observed after treatment of descending TAAs within the Society for Vascular Surgery Vascular Quality Initiative registry37. Despite adjustment for ASI, women with TAAs also remain at greater risk of dissection8. Further investigation of mechanisms underlying differences in outcomes for women and men are required, so that inequalities can be addressed in current risk stratification, surveillance, and treatment strategies.
The results of the ETTAA study should be applicable to countries with similar populations and services to the UK, as 30 cardiac centres participated and there were few patient exclusion criteria. Data collection was prospective and mandated variables were reasonably complete and checked for errors. Registration with electronic health records ensured mid- to long-term follow-up (3.5–8 years). Robust statistical methods were used, including consideration of competing risks and differences between those undergoing intervention and those in conservative management groups.
The ETTAA study is based on a heterogeneous population of patients with TAAs and analysis stratified by sex and size results in few deaths in some strata. This was particularly difficult when assessing post-intervention risks, as death may result from operative complications, aneurysm features, co-morbidity, or a combination of these. Causes of death for patients who died during the ETTAA study were checked with hospitals, whereas deaths occurring after the ETTAA study finished were available from routinely recorded national registry data only and were more likely to be misclassified38. This may partly account for the weaker patterns in the analysis of survival for AR death. Lastly, it cannot be ruled out that some selection bias occurred during recruitment. Further research using routine electronic health records could provide insight into differences in age and other characteristics at referral to cardiac services between the sexes.
Risk stratification by a size-adjusted index (for example ASI or AHI) is more successful at reducing inequities in outcomes between women and men than stratification by aneurysm diameter. Selection of a surgical threshold based on ASI or a related measurement should be strongly considered during the construction of future clinical guidelines.
Supplementary Material
Acknowledgements
The authors would like to acknowledge everyone in the ETTAA Collaborative Group (as listed in Sharples et al.14), including the Trial Steering Committee, the Data Monitoring Committee, the Principal Investigators, Research Coordinators, and Administrators at collaborating sites, and the Core ETTAA Working Group, as well as the patients who participated.
Contributor Information
Anna L Pouncey, Department of Surgery and Cancer, Imperial College London, St Mary’s Hospital, London, UK.
Dhvni Patel, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
Carol Freeman, Papworth Trials Unit Collaboration, Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK.
Priya Sastry, Department of Cardiac Surgery, John Radcliffe Hospital, Oxford, UK.
Colin Bicknell, Department of Surgery and Cancer, Imperial College London, St Mary’s Hospital, London, UK.
Stephen R Large, Department of Cardiac Surgery, Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK.
Linda D Sharples, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
Funding
This work was supported by the National Institute for Health and Care Research (Health Technology Assessment, 11/147/03—Effective Treatments for Thoracic Aortic Aneurysms (ETTAA study): a prospective cohort study). D.P. was supported by a National Institute for Health and Care Research (NIHR) pre-doctoral fellowship and A.L.P. was supported by an NIHR doctoral fellowship, when completing this work. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The ETTAA Working Group supports the principles of data sharing. Applications to access data should be submitted via the corresponding author. Access to anonymized data may be granted after review.
Author contributions
Anna L. Pouncey (Conceptualization, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Dhvni Patel (Conceptualization, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Carol Freeman (Conceptualization, Data curation, Resources, Writing—review & editing), Priya Sastry (Conceptualization, Investigation, Writing—review & editing), Colin Bicknell (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Stephen R. Large (Funding acquisition, Investigation, Project administration, Supervision, Writing—review & editing), and Linda D. Sharples (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing)
References
- 1. Johansson G, Markström U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg 1995;21:985–988 [DOI] [PubMed] [Google Scholar]
- 2. Gouveia e Melo R, Silva Duarte G, Lopes A, Alves M, Caldeira D, Fernandes E et al. Incidence and prevalence of thoracic aortic aneurysms: a systematic review and meta-analysis of population-based studies. Semin Thorac Cardiovasc Surg 2022;34:1–16 [DOI] [PubMed] [Google Scholar]
- 3. Sharples L, Sastry P, Freeman C, Bicknell C, Da Chiu Y, Vallabhaneni SR et al. Aneurysm growth, survival, and quality of life in untreated thoracic aortic aneurysms: the effective treatments for thoracic aortic aneurysms study. Eur Heart J 2022;43:2356–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Juvonen T, Ergin MA, Galla JD, Lansman SL, Nguyen KH, McCullough JN et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg 1997;63:1533–1545 [DOI] [PubMed] [Google Scholar]
- 5. Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17–28 [DOI] [PubMed] [Google Scholar]
- 6. Cheung K, Boodhwani M, Chan K, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc 2017;6:e003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zafar MA, Li Y, Rizzo JA, Charilaou P, Saeyeldin A, Velasquez CA et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 2018;155:1938–1950 [DOI] [PubMed] [Google Scholar]
- 8. Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169–177 [DOI] [PubMed] [Google Scholar]
- 9. Forbes TL, Harris JR, Lawlor DK, DeRose G. Evaluation of sex differences in relative dilatation of thoracic aortic aneurysms. Eur J Vasc Endovasc Surg 2010;39:555–558 [DOI] [PubMed] [Google Scholar]
- 10. Upchurch GR, Escobar GA, Azizzadeh A, Beck AW, Conrad MF, Matsumura JS et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg 2021;73:55S–83S [DOI] [PubMed] [Google Scholar]
- 11. Riambau V, Böckler D, Brunkwall J, Cao P, Chiesa R, Coppi G et al. Editor’s Choice – Management of descending thoracic aorta diseases. Eur J Vasc Endovasc Surg 2017;53:4–52 [DOI] [PubMed] [Google Scholar]
- 12. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW, Bolen MA et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334–e482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung J, Coutinho T, Chu MWA, Ouzounian M. Sex differences in thoracic aortic disease: a review of the literature and a call to action. J Thorac Cardiovasc Surg 2020;160:656–660 [DOI] [PubMed] [Google Scholar]
- 14. Sharples L, Sastry P, Freeman C, Gray J, McCarthy A, Da Chiu Y et al. Endovascular stent grafting and open surgical replacement for chronic thoracic aortic aneurysms: a systematic review and prospective cohort study. Health Technol Assess 2022;26:1–166 [DOI] [PubMed] [Google Scholar]
- 15. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–311, discussion 312–313 [PubMed] [Google Scholar]
- 16. Boyd A, Cornish R, Johnson L, Simmonds S, Syddall H, Westbury L et al. Resource Report Understanding Hospital Episode Statistics (HES). 2018. https://www.closer.ac.uk/wp-content/uploads/CLOSER-resource-understanding-hospital-episode-statistics-2018.pdf (accessed 4 April 2023)
- 17. Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Ann Transl Med 2019;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res 2012;18:2301–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399 [DOI] [PubMed] [Google Scholar]
- 20. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010;121:e266–e369 [DOI] [PubMed] [Google Scholar]
- 22. Girardi LN, Lau C, Gambardella I. Aortic dimensions as predictors of adverse events. J Thorac Cardiovasc Surg 2021;161:1193–1197 [DOI] [PubMed] [Google Scholar]
- 23. Garcia M, Mulvagh SL, Bairey Merz CN, Buring JE, Manson JE. Cardiovascular disease in women. Circ Res 2016;118:1273–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pouncey AL, David M, Morris RI, Ulug P, Martin G, Bicknell C et al. Editor’s Choice – Systematic review and meta-analysis of sex specific differences in adverse events after open and endovascular intact abdominal aortic aneurysm repair: consistently worse outcomes for women. Eur J Vasc Endovasc Surg 2021;62:367–378 [DOI] [PubMed] [Google Scholar]
- 25. Gulati M. Improving the cardiovascular health of women in the nation. Circulation 2017;135:495–498 [DOI] [PubMed] [Google Scholar]
- 26. Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation 2012;125:1449–1472 [DOI] [PubMed] [Google Scholar]
- 27. Pouncey AL, Powell JT. Women’s lives at stake: women suffer disproportionately after abdominal aortic aneurysm repair, so what can we do about it? Eur J Vasc Endovasc Surg 2021;62:1–3 [DOI] [PubMed] [Google Scholar]
- 28. Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020;141:540–548 [DOI] [PubMed] [Google Scholar]
- 29. Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3:135–142 [DOI] [PubMed] [Google Scholar]
- 30. Harris KM, Strauss CE, Eagle KA, Hirsch AT, Isselbacher EM, Tsai TT et al. Correlates of delayed recognition and treatment of acute type A aortic dissection. Circulation 2011;124:1911–1918 [DOI] [PubMed] [Google Scholar]
- 31. Meijboom LJ, Timmermans J, Zwinderman AH, Engelfriet PM, Mulder BJM. Aortic root growth in men and women with the Marfan’s syndrome. Am J Cardiol 2005;96:1441–1444 [DOI] [PubMed] [Google Scholar]
- 32. Jondeau G, Ropers J, Regalado E, Braverman A, Evangelista A, Teixedo G et al. International registry of patients carrying TGFBR1 or TGFBR2 mutations. Circ Cardiovasc Genet 2016;9:548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sokolis DP, Iliopoulos DC. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J Mech Behav Biomed Mater 2014;34:154–164 [DOI] [PubMed] [Google Scholar]
- 34. Boczar KE, Cheung K, Boodhwani M, Beauchesne L, Dennie C, Nagpal S et al. Sex differences in thoracic aortic aneurysm growth. Hypertension 2019;73:190–196 [DOI] [PubMed] [Google Scholar]
- 35. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA et al. Hemodynamic correlates of blood pressure across the adult age Spectrum. Circulation 2010;122:1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung J, Stevens L-M, Ouzounian M, El-Hamamsy I, Bouhout I, Dagenais F et al. Sex-related differences in patients undergoing thoracic aortic surgery. Circulation 2019;139:1177–1184 [DOI] [PubMed] [Google Scholar]
- 37. Deery SE, Shean KE, Wang GJ, Black JH, Upchurch GR, Giles KA et al. Female sex independently predicts mortality after thoracic endovascular aortic repair for intact descending thoracic aortic aneurysms. J Vasc Surg 2017;66:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaudhry Z, Mannan F, Gibson-White A, Syed U, Ahmed S, Majeed A. Research outputs of England’s Hospital Episode Statistics (HES) database: bibliometric analysis. J Innov Health Inform 2017;24:329–333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ETTAA Working Group supports the principles of data sharing. Applications to access data should be submitted via the corresponding author. Access to anonymized data may be granted after review.