Abstract
A minority of children born small for gestational age (SGA) may experience catch-up growth failure and remain short in adulthood. However, the underlying causes and mechanisms of this phenomenon are not yet fully comprehended. We reviewed the present state of research concerning the growth hormone-insulin-like growth factor axis and growth plate in SGA children who fail to achieve catch-up growth. Additionally, we explored the factors influencing catch-up growth in SGA children and potential molecular mechanisms involved. Furthermore, we considered the potential benefits of supplementary nutrition, specific dietary patterns, probiotics and drug therapy in facilitating catch-up growth.
Keywords: Small for gestational age, Catch-up growth, Growth hormone-insulin-like growth factor axis, Growth plate, Short stature
Introduction
Small for gestational age (SGA) is generally defined as the birth weight and/or birth length of infants that is less than 2 standard deviation scores (SDS) from the mean for gestational age [1]. Based on the reference data, neonates can be subdivided into SGA for weight, SGA for length, or SGA for both weight and length, which helps to understand the mechanisms and effects of being bore SGA [1]. Noteworthy, birth weight is currently the most often used reference data to define SGA newborns.
Catch-up growth in linear growth refers to “a height velocity above the statistical limits of normality for age and(or) maturity during a defined period, following a transient period of growth inhibition” [2]. The typical growth pattern of SGA children is defined as a period of accelerated linear growth, which occurs mainly in the first 12 months of life, with a complete recovery in height at the age of 2 years [3]. However, a landmark study by Karlberg et al. in 1995 reported that 10–15% of full-term SGA children lack catch-up growth, and most of these children remain short in adulthood [4]. For late preterm SGA children, one-third of them were below the 10th percentile for length at 36 months of corrected age [5]. Currently, the treatment of recombinant human growth hormone (rhGH) has been approved by many countries for short children born SGA. However, the treatment is less effective than in patients with growth hormone deficiency (GHD) [6]. In addition, the timing of initiating growth hormone therapy is controversial due to the uncertainty of spontaneous catch-up growth in children with SGA [7]. This variability may be attributable to multiple genetic variations and other causes of perturbation of linear growth [7, 8]. Therefore, exploring the mechanisms affecting insufficient linear catch-up growth is necessary for optimizing the adult height of short SGA children. Although several studies on SGA children describe catch-up growth in both height and weight, we focus on catch-up growth in height in this review.
Here, we review the role of the growth hormone-insulin-like growth factor axis and the growth plate in SGA children lacking catch-up growth, the numerous factors affecting catch-up growth, and the possible molecular mechanisms. Lastly, we conclude with a discussion of potential therapeutic approaches for short SGA children.
Main text
Etiology of SGA
Maternal, placental, and fetal factors interact in a complicated way to cause the multifactorial etiology of SGA [1, 9]. Maternal nutrition, health status, medications, habits, and genetic factors (e.g., height, weight, and uterine capacity) contribute to the growth of the fetus. Placental insufficiency and anomalies prevent the placenta from providing an appropriate quantity of nutrition and oxygen to the fetus, which most frequently occurs in pre-eclampsia. Fetal factors such as multiple genetic syndromes and perinatal infections may affect offspring birth size.
The etiology of SGA may affect catch-up growth. Fang et al. classified the probable causes of SGA into five etiologic subgroups, and investigated the effect of each group on the risk of growth restriction in children with SGA at age 7 years for the first time [10]. This study concluded that low maternal height was a physiological factor, while smoking during pregnancy was an environmental factor. SGA patients with mothers whose heights were less than the third percentile had the highest risk of short stature at age 7 years (the adjusted odds ratio (aOR) = 6.04, 95% CI 3.93–9.27). The fetal etiology group came in second, with emphasis on growth limitation at age 7 years due to serious birth defects such as congenital heart disease, maternal viral infections during pregnancy, Down syndrome, and major birth defects that may influence fetal growth and development. (aOR = 4.78, 95% CI 3.41–6.71) [10]. SGA children with unknown risk factors, maternal factors, placental factors, and environmental factors also had a higher risk of growth restriction at 7 years. (aOR = 3.84, 95% CI 2.64–5.58, aOR = 3.47, 95% CI 2.62–4.60, aOR = 3.36, 95% CI 2.51–4.46, aOR = 2.19, 95% CI 1.59–3.03, respectively) [10]. Unknown risk factors may indicate undiagnosed genetic defects. It is important to note that the placental etiology subgroup may be at higher risk for growth restriction than the study reflects because the most important placental factor, pre-eclampsia, was considered a maternal factor in this study.
Overall, all infants with maternal, fetal, environmental, placental, and genetic variables that contributed to SGA had a higher chance of growth limitation at age 7 years than normal appropriate for gestational age (AGA) infants. Our review will discuss and explore their respective characteristics and possible mechanisms of catch-up growth below.
Pathophysiology in short SGA children
There have been two main accepted models of catch-up growth. One is the neuroendocrine hypothesis, which was proposed by Tanner in 1960s [11]. It suggested that the hypothalamus can compare body size with the ideal size of an individual at that age (possibly encoded by a gene). When there is a mismatch, the body grows faster than average by adjusting growth factors. The other one is the growth plate hypothesis. Animal studies suggested that growth plate chondrocytes have a limited ability to proliferate. After a brief period of growth inhibition, the growth plate showed remarkable ability to proliferate; therefore, catch-up growth occurs [12]. Since then, many hypotheses have been extended from these two hypotheses [13]. Roselló-Díez et al. provided an extensive discussion on catch-up growth in 2015 [2]. It clarified that catch-up growth was a combination of the two primary recognized concepts and occurred at three levels: the cell itself, the tissue, and the collaboration between different organs. Cells have their own "growth program", after which all cells within a tissue coordinate with each other through a common program. Ultimately, the coordinated growth between different organs relies on intrinsic programs and extrinsic hormones.
The GH/IGF-1 axis
GH/IGF-1 axis plays an extremely significant role in longitudinal growth and development [14]. The function of growth hormone (GH) depends on the growth hormone receptor (GHR). GHR affects insulin-like growth factor 1 (IGF-1) gene expression and thus leads to IGF-1 secretion [15]. Most circulating IGF-1 is produced by the liver and is regulated prenatally by insulin and postnatally by GH. Circulating IGF-1 binds mainly to IGF-binding protein 3 (IGFBP-3) and IGF-binding protein 5 (IGFBP-5), which in turn form a ternary complex with acid-labile subunit (ALS), significantly prolonging the half-life of the circulating IGF1-IGFBP binary complex [16]. IGF-1 acts by binding to IGF-1 receptors (IGF1R). IGF1R binds to the ligand, leading to autophosphorylation and tyrosine phosphorylation. Subsequently, substrate phosphorylation activates two major signaling pathways, the phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (AKT)/mammalian target of rapamycin (mTOR) and RAS/mitogen-activated protein kinase (MAPK) [17]. After that, GH-IGF-mTOR/MAPK pathway can be activated by multiple factors such as nutrition [18]. Activation of the GH-IGF-mTOR/MAPK pathway initiates numerous fundamental cellular processes in organ and body growth and development [19, 20]. In growth plates, GH-IGF-mTOR pathway is responsible for the linear growth of the long bones [19].
In the intrauterine period, fetal growth retardation is manifested by reduced insulin and IGF-1 levels [21]. After birth, it was reported that the SGA neonates showed resistance to GH and IGF-1 compared to those AGA infants [22]. Then growing up for a while, the short SGA children also rarely developed typical GH deficiency, but there were abnormalities in the GH/ IGF axis [23]. (Fig. 1).
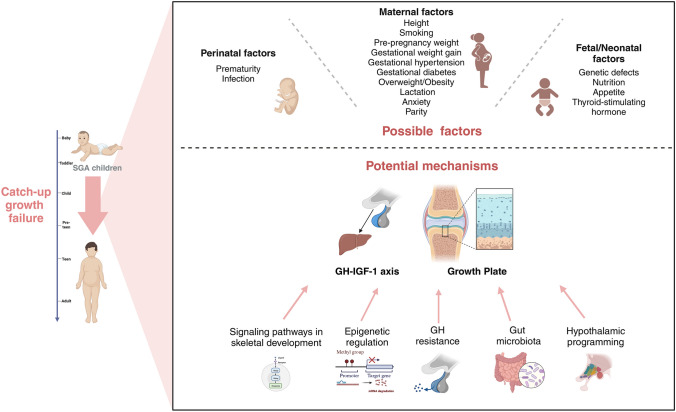
Fig. 1.
The possible factors and potential mechanisms of catch-up failure in SGA children. Disorders of the GH-IGF-1 axis and abnormal cartilage ossification within the growth plate are the main pathological features of short SGA children. Multiple maternal, perinatal, neonatal and fetal factors impaired the ability of SGA infants to catch up. Signaling pathways in skeletal development, epigenetic regulation, GH resistance, gut microbiota, and the reprogramming of the hypothalamic–pituitary–adrenal axis are the possible mechanisms for catch-up growth failure
Several studies revealed that, as compared to controls, persistently short SGA children exhibited lower spontaneous GH secretion [24]. In particular, short SGA children under 6 years of age have alternative GH secretion patterns including high basal GH levels, high peak frequencies, but low peak amplitudes [24]. This aberrant GH pattern, however, may be a phenomenon specific to a certain age range, as it ceases in children with SGA beyond the age of 6 years [25]. Overall, the current study shows that low spontaneous GH levels, rather than altered secretory rhythms, remain the primary cause of aberrant GH secretion in short SGA and this difference in secretion rates is age-related, with GH secretion rates increasing with age.
Due to lower natural GH secretion, the IGF-1 levels were much lower in the non-catch-up growth subgroup compared to the SGA children who caught up in growth [26]. This supports the hypothesis that IGF-1 and childhood height are positively associated [27]. Additionally, in children treated with rhGH, it was found that short children with SGA may have some degree of IGF-1 resistance because they required higher GH-induced IGF-I levels to achieve growth rates similar to those of children with familial short stature or GHD [22]. SGA children with this mildly aberrant IGF-1 signaling pathway are common; about half of the short children born SGA fulfill the criteria for IGF-1 resistance [28]. Indeed, the unique physiological circumstances and genetic heritage of short SGA children may be the root of the debate regarding IGF-1 levels. If SGA children showed normal GH secretion with IGF-1 resistance, they often have mutations in either the IGF1R gene or the IGF1 gene [28]. When the baseline GH level is naturally low, factors influencing IGF-1 sensitivity could be identified, such as administration of exogenous rhGH, dosage adjustments for weight gain, treatment compliance, and characteristics of the study population (including younger age, delayed bone age, extreme shortness, and partial syndromes) [29].
The growth plate
Endochondral ossification is the pathway for organogenesis and skeletal elongation. The rate of postnatal bone growth is almost entirely dependent on the cartilage growth plate [30]. The growth plate is located between the epiphysis and the metaphysis of long bones and is divided into three well-defined zones. The resting zone (RZ) closest to the epiphysis generally serves to support the proliferative zone (PZ). PZ, directly below the RZ, begins to divide and form a columnar arrangement. Then, in the hypertrophic zone (HZ), the chondrocytes initiate terminal differentiation [31].
Long bones lengthen with the proliferation and hypertrophy of growth plate chondrocytes and are closely controlled by systemic endocrine molecules. The major endocrine factors regulating the growth plate include GH, IGF-1, thyroid hormones, and so on. Indian hedgehog (IHH), parathyroid hormone-related peptide (PTHrP), and fibroblast growth factors (FGFs) are examples of autocrine/paracrine molecules that act locally in the growth plate [18, 32]. They are all responsible for helping the growth plate transition from the proliferative zone to the hypertrophic zone and function correctly [32]. The rabbit model demonstrated that during catch-up growth, the proliferative zone, hypertrophic zone, and total growth plate all experienced a delayed decline in senescence. This suggests that, at least in part, the delay in growth plate senescence is what allows for greater proliferative capacity during linear catch-up growth. By modifying the cellular senescence process, it might be feasible to encourage catch-up growth in SGA children more successfully [33].
Recent studies suggested that SGA children with inadequate catch-up growth had abnormal skeletal development. SGA infants had significantly lower femur, tibia, humerus lengths and cortical bone mass than AGA infants [34]. When SGA children experience catch-up growth in height postnatally, they can reach the average long bone length and mineralization by 4 years of age [35, 36]. Still, SGA children who lack catch-up growth have insufficient bone mineral accumulation during growth and are at risk for low adult bone mass [37]. Cohort studies highlight the bone mineralization is the outcome of catch-up growth. Hormone level improvements alone, such as exogenous rhGH injection, are not sufficient to promote bone formation in short SGA cases. Research by Schweizer et al. revealed that rhGH treatment did not increase bone diameter in children with short SGA [38, 39]. The above-mentioned studies showed that regulatory problems at two levels—growth plate senescence control and endocrine system hormone regulation—are the cause of catch-up failure in SGA children. The limitation of the study is that the subjects were only full-term SGA children. In children with preterm SGA, a severe prenatal shock may have a direct impact not only on growth catch-up but also on bone formation.
Multiple influencing factors in inadequate catch-up growth in SGA children
Maternal factors
Maternal factors not only cause neonates to exhibit SGA at birth, but further affect postnatal catch-up growth in SGA children. These determinants cover not only maternal physiological characteristics (e.g., height, weight, weight gain during pregnancy, placental function, and illnesses during pregnancy), but also maternal psychological states (e.g., anxiety, depression) and breastfeeding behaviors. A study analyzing a subgroup of the Early Childhood Longitudinal Study Birth Cohort (ECLS-B) found that the effects of short maternal height, pre-pregnancy underweight, inadequate gestational weight gain, and smoking on poor catch-up growth before school age [7]. Placental insufficiency alters postnatal growth trajectories in very low birth weight (VLBW) children, but early placental insufficiency does not appear to affect height at 12.5 years of age in children with SGA [40, 41]. More research is therefore needed to focus on the impact of varying degrees and timing of placental insufficiency on the early growth patterns of children with SGA. Another prospective cohort study also showed that gestational hypertension, gestational diabetes, and maternal overweight/obesity were inversely associated with catch-up growth in SGA children [42]. Preeclampsia, a hypertensive disorder of pregnancy, may include pathologically varied disorders that are categorized according to severity or onset [43]. The effect of preeclampsia on offspring’s longitudinal growth is heterogeneous, and there have been no investigations evaluating preeclampsia as an independent factor for catch-up growth in the SGA state [43].
Moreover, some maternal factors universally affecting postpartum growth in offspring could also have a great impact on the catch-up growth of SGA children. These factors are often associated with abnormalities in maternal lactation. An analysis of the Avon Parent–Child Longitudinal Study (ALSPAC) cohort in the United Kingdom showed that the degree of maternal anxiety was negatively associated with children's BMI in the second year of a child's life [44]. Recent researches supposed maternal depression during pregnancy causes delays in breastfeeding initiation and affects breastfeeding patterns [45, 46]. Furthermore, parity played a role in growth, as another analysis of the ALSPAC cohort showed that infants of first-time mothers go through rapid catch-up growth in weight and length during the first year of life, with significant increases in weight and length from 12 months of age [47]. Parity's effect on the timing of the initial breastfeeding might be the reason for inadequate catch-up growth in SGA children [48].
Perinatal factors
Preterm birth is another factor of insufficient catch-up growth. A community-based cohort study showed that children born preterm SGA had an increased likelihood of postnatal growth restriction compared to full-term SGA children and preterm AGA children. About 30–39% of preterm SGA children aged four could not catch up on growth, compared with only 5% of preterm AGA children and 9% of term SGA children who do not catch up [49]. The study revealed that premature birth and SGA status jointly affect growth potential, which was likely due to the neonatal complications and chronic diseases related with preterm birth, affecting early growth [50, 51].
Perinatal infection also causes catch-up growth failure in SGA children. Congenital infections caused by Toxoplasma gondii, rubella, cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus (VZV), and Treponemes not only cause SGA in newborns, but may also further affect its postpartum growth potential [52]. An observational study found that newborns with SGA with CMV infection have slightly different growth patterns than those with general SGA. Typically, 90% of SGA tend to experience accelerated catch-up growth in the first two years. However, the catch-up rate among SGA children with CMV infection is about 75% [53]. Multiple causes contribute to the failure of catch-up growth in SGA children with CMV infection. Children with severe neurologic disease caused by CMV are often associated with nutritional difficulties and endocrine disorders, such as abnormalities in GH secretion. Second, poor motor function due to CMV may affect bone growth in children with SGA. In addition, CMV may play a direct role as a pathogen in catch-up growth failure [53].
Fetal/neonatal factors
Molecular genetic analyses revealed that many short SGA children exhibit genetic defects. The international consensus guide on SGA children, published in 2023, is an important compilation of the genetic reasons for SGA children's short stature [1]. A genetic analysis of 176 SGA children with persistent short stature showed that 42% of them had pathogenic or potentially pathogenic genetic variants (P/LP) [54]. Of all the variants detected, the most significant number of variants were those related to the growth plate, accounting for 42% of the total, with 7/74 children having variants in the short stature homeobox (SHOX) gene, 7/74 having variants in paracrine signaling of the growth plate, and 17/74 having variants in genes related to the cartilaginous extracellular matrix of the growth plate. 16% of P/LP gene variants affect the pituitary, GH-IGF-1, and IGF-2 axes. 3% of the P/LP gene variants affected the thyroid axis, as evidenced by TRHR and THRA variants. In 12/74 children (16%), the study also identified P/LP variants in genes involved in fundamental intracellular and intranuclear processes. The diagnosis of Silver-Russell syndrome (11p15, UPD7) was seen in 12/74 (16%) of cases, and other chromosomal aberrations were seen in 5/74 (7%) of children [54]. Genetic variations typically affect three key aspects of growth regulation: the endocrine system, growth plate function, intracellular regulation, and signal transduction processes. These align with the three regulatory levels described by the catch-up growth theory. However, the next-generation sequencing (NGS) used in research cannot identify epigenetic alterations. Hence, toxicant-induced epigenetic changes caused by maternal factors, such as smoking, alcohol consumption, and infectious diseases during pregnancy, cannot be detected.
As far as postnatal factors are concerned, nutrition and appetite play an extremely important role. Epidemiological evidence suggested that malnourished SGA children were more likely to exhibit failure to catch up on growth. Catch-up growth failure is primarily caused by malnutrition due to inappropriate breastfeeding, lack of complementary foods, infections, and other environmental factors [55]. Moreover, appetite could also be the reason for insufficient catch-up growth. Gastrin and leptin are crucial hormones that regulate appetite, food intake, and energy metabolism. Previous studies revealed that leptin and gastrin levels in the umbilical cord have predictive value for postnatal catch-up growth [56, 57]. These findings supported the possibility that satiety levels preset by the uterus may partially mediate changes in infant growth rate [58].
Average somatic growth requires that the thyroid hormone axis and the GH axis work together, and abnormalities in the thyroid hormone axis affect the GH-IGF-1 axis [59]. Postnatal thyroid stimulating hormone (TSH) in SGA children was negatively associated with postnatal catch-up growth [60, 61].
Among all the factors discussed above, genetic and epigenetic factors play major roles in postnatal catch-up growth failure, as evidenced by the fact that some SGA children have monogenic disorders, genetic defects, or epigenetic disorders such as Silver-Russell syndrome [1]. Secondly, maternal height is undoubtedly a crucial factor, which represents the limited genetic potential for linear growth in newborns and can affect the catch-up growth ability of SGA children. However, the heritability of height velocity throughout infancy is not fully understood [62]. Furthermore, in low- and middle-income nations, population height variations are more likely to be attributable to environmental factors than to genetics. Nutrition and disease are the main environmental factors affecting height [63]. For linear growth in children, intrauterine development and exposure before the age of two years are the main drivers. Therefore, physiologic conditions during pregnancy and health management during the neonatal period are particularly important for catch-up growth in children with SGA [64]. After emphasizing the magnitude of each factor, we stress that the number of SGA risk factors has a multiplier effect on children's postnatal growth. Xie et al. found that experiencing "multiple hits" in utero resulted in more severe outcomes. For example, SGA newborns with both maternal smoking and inadequate gestational weight gain during pregnancy have a much higher risk of stunting at the age 5 [7, 10].
Potential molecular mechanisms
Signaling pathways in skeletal development
Genetic investigations of children born SGA with persistent short stature revealed that the majority of children in this group had genetic variations linked to growth plate development [65]. Hence, correct growth plate function is essential for SGA children to catch up on growth.
The process of forming growth plates is dynamic and strictly controlled. Numerous signaling pathways interact and are crucial to this process. The primary signaling pathways are Transforming Growth Factor (TGF)/Bone Morphogenetic Protein (BMP), FGF, WNT/β-catenin, Hedgehog, PTHrP, and notch signaling [66]. The signaling cascades converge on chondrocyte transcription factors. Runx2, a member of the Runt family of structural domains; Osterix, a member of the SP/KLF family; and Sox9, a member of the Sox family of transcription factors, all play crucial roles as transcription factors in cartilage production [66, 67]. Additionally, a number of transcriptional cofactors, including β-connexin, CBF, JAB1, and YAP1, have been demonstrated to be essential for the development of growth plates [66]. SGA children with catch-up failure exhibit not only P/LPs implicated in FGF and IHH signaling pathways, but also P/LPs influencing basic intracellular/intranuclear processes (CDC42, KMT2D, LMNA, NSD1, PTPN11, SRCAP, SON, SOS1, SOX9. TLK2) [54, 65]. Unfortunately, no comprehensive investigations of skeletal development in children with dwarf SGA have been conducted. CXXC finger protein 5 (CXXC5), a negative feedback regulator of WNT/β-catenin, has been shown to mediate growth plate senescence in a mouse model and is a potential target for enhancing longitudinal bone growth, offering new possibilities for diagnosis and treatment [68]. However, the expression of CXXC5 in the growth plate only increases progressively during late puberty, and its role in catch-up growth, which occurs mainly in the first two years of life, remains unclear [68]. More research is needed into the involvement of intracellular/intranuclear signaling in growth plate signaling pathways, as well as the mechanisms involved in catch-up growth failures.
GH resistance
The phenomenon of GH resistance has been described in short children with SGA undergoing rhGH treatment, evidenced by poorer improvements in bone strength and muscular mass compared to those with GHD [38]. Furthermore, a cohort of SGA children treated with rhGH showed varied improvements in motor function, further reflecting heterogeneity in GH sensitivity [69]. The heterogeneity in dwarf SGA patients may stem from a long-term nutritional imbalance [70]. For example, a lack of protein or other dietary components during the first two years of life can cause GH resistance [70]. In addition, lower insulin and leptin levels due to nutritional deprivation may partially mediate GH resistance by downregulating GH receptors in the liver [70]. The compensation growth pattern can explain the catch-up growth failure. GH and ghrelin levels were elevated due to low cellular nutrient levels, while IGFs and leptin were suppressed. The adverse environment increased nicotinamide adenine dinucleotide (NAD +) and adenosine monophosphate (AMP), which in turn stimulated the production of NAD-dependent deacetylase sirtuin-1 (SIRT1) and fibroblast growth factor 21 (FGF21). SIRT1 and FGF21 blocked the Janus-activated kinase 2 (JAK2) and signal transducer and activator of the transcription 5 (STAT5) signaling pathway, ultimately resulting in hepatic GH resistance [71–74]. Animal models suggested that key regulators in cytokine signaling pathways may alter downstream signaling pathways of GH, independent of postnatal nutrition. A correlation between the absence of catch-up growth and hepatic GH resistance was also observed in the uterine artery ligation-induced intrauterine growth retardation (IUGR) rat model. In addition to impaired GH-mediated JAK2/STAT5 signaling, IUGR rats without catch-up growth were found to have up-regulation of GH-induced suppressor of cytokine signaling (SOCS/CIS proteins) [75]. Therefore, in addition to postnatal nutritional deprivation, a poor intrauterine environment could lead to GH resistance by modulating SOCS/CIS expression, which further impair the offspring's catch-up growth.
Epigenetic regulation
Epigenetic regulation may modulate the somatotropic axis in short SGA children and result in catch-up growth failure. Pregnant women who smoke, drink alcohol, and have infectious diseases during pregnancy may result in toxicant-induced epigenetic alterations in the fetus [7]. Epigenetics refers to changes in gene expression levels due to non-genetic sequence alterations, primarily including regulation of DNA methylation, chemical modification of histones, and non-coding RNA expression [76]. Genomic imprinting is one of the most important and well-studied forms of the epigenetic phenomenon in short SGA children, which causes genes to be expressed in a parent-of-origin-specific manner. Silver-Russell syndrome (SRS), mainly driven by IGF2 / H19 imprinting domain hypomethylation in the 11p15 region, is characterized by IUGR and short stature due to catch-up growth failure [77]. However, 11p15 epigenetic mutations are rare in SGA children without Silver-Russell syndrome [78].
Methylation disorders in children born with SGA may present not only in imprinted loci but also in non-imprinted genes [9, 79]. A key characteristic of genes regulated by epigenetic modifications is their involvement in growth and development. The majority of SGA children with aberrant methylation fail to catch up on growth [79]. Animal experiments showed that prenatal exposure to inflammation leads to hypomethylation of Mecp2 and LINE1 in the mouse hypothalamus, which may further affect the neuroendocrine system and growth potential [80].
MicroRNAs (miRNAs) may also contribute to SGA children's insufficient catch-up growth. Jeong et al. used NGS to analyze serum exosome miRNAs from 16 SGA and 10 AGA children. They found that four upregulated miRNAs: miR-30c-5p, miR-363-3p, miR-29a-3p, miR-29c-3p, and two downregulated miRNAs: miR-629-5p and miR-23a-5p were involved in SGA children without catch-up growth, and all these miRNAs were associated with cell proliferation, cell growth, IGF-1R regulation or aging process [81–83]. Mas-Parés et al. showed umbilical cord miRNAs could be novel biomarkers for the early identification of catch-up growth in SGA infants: miR-501-3p, miR-576-5p, miR-770-5p, and miR-876-3p may contribute to the regulation of postnatal height growth [84].
Gut microbiota
In a study of Hispanic infants in Southern California, Alderete et al. found that a more mature gut microbiota at one month of age, characterized by increased alpha diversity, predicted catch-up growth [85]. Studies on preterm infants also indicated that the acquisition of gut microbiota was associated with optimal growth trajectories [86]. Additionally, gut microbiota composition differed between SGA rats with and without catch-up growth, suggesting that differences in microbiota may contribute to SGA children's differences in postnatal growth potential [87].
Gut microbiota regulates chondrogenic ossification and the GH-IGF-1 axis in complex ways. By enhancing peripheral tissue sensitivity to GH and raising circulating levels of IGF-1 in mice, the strains of bacterium Lactobacillus plantarum promoted the GH axis, overcoming chronic malnutrition-induced GH resistance and developmental delay [88]. Recent research revealed that the cell wall of Lactobacillus plantarum enhanced the GH-IGF-1 axis by increasing Nucleotide-binding oligomerization domain-containing protein 2(NOD2) signaling. Malnutrition inhibited the proliferation of small intestinal crypt cells. Activation of NOD2 signaling by Lactobacillus plantarum cell wall increases intestinal cell proliferation and improves nutrient absorption, thereby stimulating the activity of the nutrient-sensitive GH/IGF-1/insulin axis and promoting postnatal growth [89]. The length of mice's femurs also grew postnatally, indicating the importance of the gut microbiota in skeletal development. Consistently, Yan et al. demonstrated that the gut microbiota may regulate endochondral ossification by increasing IGF-1 levels through the production of short-chain fatty acids (SCFAs, a byproduct of the gut microbiota), thereby altering longitudinal bone growth [90]. SCFAs could also improve gut barrier function and create an anti-inflammatory environment, and their role in catch-up growth in children with SGA warrants further exploration [90–92].
Reprogramming of the hypothalamic–pituitary–adrenal axis
A study of 49 children with IUGR suggested that catch-up growth in children with IUGR may be influenced by intrauterine reprogramming of the HPA axis, and children with increased cortisol secretion may have a higher likelihood of growth failure [93]. Factors including exposure to xenobiotics and psychosocial stress throughout pregnancy, may lead to intrauterine programming and change the hypothalamic–pituitary–adrenal (HPA) axis. Exogenous substances may cause programmed alterations in the fetal HPA axis via epigenetic modifications of essential genes or oxidative stress in the fetal adrenal glands [94]. However, cortisol which is lipophilic and can pass through the placenta, is usually considered the primary mediator. Excess maternal cortisol may continue to impair fetal HPA axis development and result in growth failure [94, 95]. In rats, maternal undernutrition could induce IUGR and overexpose the fetus to maternal corticosterone, leading to increased cortisol secretion in newborns and potential growth retardation [96]. In monkeys, dexamethasone treatment during pregnancy caused a reduction in hippocampal volume and an increase in postnatal plasma cortisol levels. The HPA axis was also enhanced postnatally in fetuses of ewes that were malnourished during the first half of gestation [93, 97]. Because of the hyperactivity of the HPA axis induced by numerous circumstances, elevated cortisol may act by limiting the proteolysis of IGFBP-3, thereby reducing the bioavailability of IGFs [93]. Through what molecular mechanisms intrauterine reprogramming of HPA axis regulates the IGF system to participate in postnatal growth retardation in SGA children will be the focus of future studies.
Potential therapeutic approaches
Several studies showed that rhGH therapy effectively induced catch-up growth in children with SGA, accompanied by normal body proportions, and improved adult height in the majority of short SGA children [98–100]. However, the growth response to GH was highly variable in all clinical trials involving short children born SGA, and this variability may be attributable, at least in part, to multiple genetic variations [8].
Currently, few studies address the treatment of inadequate catch-up growth among SGA children due to the unknown mechanism behind it. Some animal studies, however, have focused on providing additional nutrients in food restriction models to induce catch-up growth. Based on the similarities in GH resistance and abnormal growth plate development between the food restriction model and the SGA model lacking catch-up growth, these treatments may be applicable to SGA children lacking catch-up growth [101]. Various studies revealed that additional nutritional supplements could lead to catch-up growth in refed animal models by promoting bone growth. In the nutritional restriction and refed rat model, the height of the growth plate fed with casein and whey protein was higher than that provided with a regular diet, and the bone strength and growth rate of rats fed with casein were higher than those fed with whey protein. Higher calcium absorption, induction of IGF-1 secretion, alteration in amino acid profile and digestion velocity may account for this phenomenon [102]. For instance, β Palmitate, the most abundant saturated fatty acid in human milk, was also reported to increase the tibia length and growth plate in refed rats [103]. In addition, a slowly digestible carbohydrate (SDC) diet can improve bone mineral density (BMD), bone mineral content (BMC), growth plate width of limbs, and middle axis bone in a refed rat model [104]. These results suggested that specific dietary patterns and additional nutritional supplements could promote the effects of catch-up growth.
Pharmaceuticals targeting specific protein or gene could be beneficial for SGA children with genetic defects. Recombinant human C-type natriuretic peptide (CNP) analogs are currently authorized in the European Union to treat chondrodysplasia [105]. The fibroblast growth factor receptor three gene (FGFR3), a negative bone growth regulator that signals through several different pathways, is the cause of chondrodysplasia. A signaling through the MAPK pathway appears to be the most significant factor in the inhibition of bone growth [106]. CNP is a selective agonist of the natriuretic peptide receptor (NPR2). NPR2 predominantly inhibits MAPK signaling in the growth plate, which speeds up chondrocyte division, matrix production, and cellular hypertrophy [106]. In order to address the decreased chondrogenesis observed in individuals with short stature, Lui et al. created a cartilage-targeted single-chain human antibody fragment (CaAb) designed to deliver therapeutic molecules to the growth plate. In a mouse model with GHD, the subcutaneous injection of the CaAb-IGF-1 fusion protein resulted in an overall increase in growth plate height, while not affecting the proliferation of renal cortical cells, thus minimizing off-target effects on non-cartilage tissues [107]. Given that some short SGA children have similar pathway abnormalities, the therapeutic effect of pharmaceuticals targeting treatments on short SGA children warrants deeper study.
Conclusions
The mechanisms underlying the absence of catch-up growth in children born with SGA still need to be better understood—several maternal, perinatal and fetal factors affect the ability of SGA infants to catch up. Genetic defects are the most important explanation for the absence of catch-up growth, with signaling pathways in skeletal development, GH resistance, reprogramming of the hypothalamic–pituitary–adrenal axis, epigenetics, and the gut microbiome as possible mechanisms. Ultimately, SGA offspring have impaired postnatal catch-up growth, which increases the risk of dwarfism in adulthood.
Although GH therapy is currently effective in reducing the risk of stunting in most cases of short SGA, the response to GH varies greatly across all clinical trials, and the optimal time to begin GH therapy is debatable due to the uncertain timing of catch-up growth [7–9]. In order to intervene earlier and reduce the likelihood of insufficient catch-up growth, more study on the etiology is required. In terms of other prospective treatment techniques, the idea of translating data from animal trials to clinical application should be pursued.
For decades, there has been little research into the causes and potential mechanisms of catch-up development failure in SGA children. Understanding the etiology and probable causes of catch-up growth failure in SGA children is therefore critical for early detection and treatment options.
Abbreviations
- ALS
Acid-labile subunit
- AMP
Adenosine monophosphate
- aOR
Adjusted odds ratio
- AGA
Appropriate for gestational age
- ALSPAC
Avon Parent–Child Longitudinal Study
- BMC
Bone mineral content
- BMD
Bone mineral density
- BMP
Bone Morphogenetic Protein
- CaAb
Cartilage-targeted single-chain human antibody fragment
- CI
Confidence Interval
- CNP
C-type natriuretic peptide
- CXXC5
CXXC finger protein 5
- CMV
Cytomegalovirus
- ECLS-B
Early Childhood Longitudinal Study Birth Cohort
- FGF21
Fibroblast growth factor 21
- FGFR3
Fibroblast growth factor receptor three gene
- FGFs
Fibroblast growth factors
- GH
Growth hormone
- GHD
Growth hormone deficiency
- GHR
Growth hormone receptor
- HGF
Hepatocyte growth factor
- HSV
Herpes simplex virus
- HZ
Hypertrophic zone
- HPA
Hypothalamic-pituitary-adrenal
- IGF1R
IGF-1 receptors
- IGFBP-3
IGF-binding protein 3
- IGFBP-5
IGF-binding protein 5
- IHH
Indian hedgehog
- IGF-1
Insulin-like growth factor 1
- IUGR
Intrauterine growth retardation
- JAK2
Janus-activated kinase 2
- mTOR
Mammalian target of rapamycin
- miRNAs
MicroRNAs
- MAPK
Mitogen-activated protein kinase
- NPR2
Natriuretic peptide receptor 2
- NGS
Next-generation sequencing
- NAD +
Nicotinamide adenine dinucleotide
- NOD2
Nucleotide-binding oligomerization domain-containing protein 2
- PTHrP
Parathyroid hormone-related peptide
- P/LP
Pathogenic or potentially pathogenic
- PZ
Proliferative zone
- AKT
Protein kinase B
- PI3K
PTHrP Phosphatidylinositol 3-kinase
- rhGH
Recombinant human growth hormone
- RZ
Resting zone
- SHOX
Short stature homeobox
- SCFAs
Short-chain fatty acids
- STAT5
Signal transducer and activator of the transcription 5
- SRS
Silver-Russell syndrome
- SIRT1
Sirtuin-1
- SDC
Slowly digestible carbohydrate
- SGA
Small for gestational age
- SDS
Standard deviation scores
- TSH
Thyroid stimulating hormone
- TGF
Transforming Growth Factor
- VZV
Varicella-zoster virus
- VLBW
Very low birth weight
Authorship contributions
AT: conceptualization and writing – original draft. FM, SL and YW: data curation and literature formation. CZ and XL: conceptualization, writing – review & editing, and supervision. All authors read and approved the final manuscript.
Funding
This study was supported by the Knowledge Innovation Program of Wuhan-Shuguang Project (No.2022020801020449).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not needed for this review article.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Key Messages
Disorders of the growth hormone-insulin-like growth factor axis and abnormal cartilage ossification within the growth plate are the main pathological features of short SGA children. There is growing evidence that maternal, perinatal and offspring’s factors influence catch-up growth in children with SGA. A number of potential molecular mechanisms may link these factors to the pathological features. Exploration of the etiology and possible mechanisms could facilitate early detection of catch-up growth failure in children with SGA and early intervention. Finally, we discuss new therapeutic approaches for the future.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cai Zhang, Email: caizhang@tjh.tjmu.edu.cn.
Xiaoping Luo, Email: xpluo@tjh.tjmu.edu.cn.
References
- 1.Hokken-Koelega ACS, van der Steen M, Boguszewski MCS, Cianfarani S, Dahlgren J, Horikawa R, et al. International consensus guideline on small for gestational age: etiology and management from infancy to early adulthood. Endocr Rev. 2023;44(3):539–65. 10.1210/endrev/bnad002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roselló-Díez A, Joyner AL. Regulation of long bone growth in vertebrates; it is time to catch up. Endocr Rev. 2015;36(6):646–80. 10.1210/er.2015-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J, Jiang Y, Huang J, Zhang Y, Zhang Y, Zhang Y, et al. Postnatal growth of preterm infants during the first two years of life: catch-up growth accompanied by risk of overweight. Ital J Pediatr. 2021;47(1):66. 10.1186/s13052-021-01019-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38(5):733–9. 10.1203/00006450-199511000-00017 [DOI] [PubMed] [Google Scholar]
- 5.Vizzari G, Morniroli D, Tiraferri V, Macchi M, Gangi S, Consales A, et al. Postnatal growth of small for gestational age late preterm infants: determinants of catch-up growth. Pediatr Res. 2023;94(1):365–70. 10.1038/s41390-022-02402-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Shaikh A, Daftardar H, Alghamdi AA, Jamjoom M, Awidah S, Ahmed ME, et al. Effect of growth hormone treatment on children with idiopathic short stature (ISS), idiopathic growth hormone deficiency (IGHD), small for gestational age (SGA) and Turner syndrome (TS) in a tertiary care center. Acta Biomed. 2020;91(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie C, Epstein LH, Eiden RD, Shenassa ED, Li X, Liao Y, et al. Stunting at 5 years among SGA newborns. Pediatrics. 2016;137(2): e20152636. 10.1542/peds.2015-2636 [DOI] [PubMed] [Google Scholar]
- 8.de Graaff LC, Clark AJ, Tauber M, Ranke MB, Johnston LB, Caliebe J, et al. Association analysis of ten candidate genes in a large multinational cohort of small for gestational age children and children with idiopathic short stature (NESTEGG study). Horm Res Paediatr. 2013;80(6):466–76. 10.1159/000355409 [DOI] [PubMed] [Google Scholar]
- 9.Finken MJJ, van der Steen M, Smeets CCJ, Walenkamp MJE, de Bruin C, Hokken-Koelega ACS, et al. Children born small for gestational age: differential diagnosis, molecular genetic evaluation, and implications. Endocr Rev. 2018;39(6):851–94. 10.1210/er.2018-00083 [DOI] [PubMed] [Google Scholar]
- 10.Fang F, Chen Y, Chen Q, Li J, Luo Z-C, Li F, et al. Etiological subgroups of term small-for-gestational-age and childhood health outcomes. Pediatr Res. 2023;94(1):378–84. 10.1038/s41390-022-02412-1 [DOI] [PubMed] [Google Scholar]
- 11.Tanner JM. Regulation of growth in size in mammals. Nature. 1963;199:845–50. 10.1038/199845a0 [DOI] [PubMed] [Google Scholar]
- 12.Emons JA, Boersma B, Baron J, Wit JM. Catch-up growth: testing the hypothesis of delayed growth plate senescence in humans. J Pediatr. 2005;147(6):843–6. 10.1016/j.jpeds.2005.07.033 [DOI] [PubMed] [Google Scholar]
- 13.Jansson JO, Dalmau Gasull A, Schéle E, Dickson SL, Palsdottir V, Palmquist A, Gironès FF, Bellman J, Anesten F, Hägg D, Ohlsson C. A body weight sensor regulates prepubertal growth via the somatotropic axis in male rats. Endocrinology. 2021;162(6):bqab053. [DOI] [PMC free article] [PubMed]
- 14.Rosello-Diez A, Joyner AL. Regulation of long bone growth in vertebrates; it is time to catch up. Endocr Rev. 2015;36(6):646–80. 10.1210/er.2015-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storr HL, Chatterjee S, Metherell LA, Foley C, Rosenfeld RG, Backeljauw PF, et al. Nonclassical GH insensitivity: characterization of mild abnormalities of GH action. Endocr Rev. 2019;40(2):476–505. 10.1210/er.2018-00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranke MB. Insulin-like growth factor binding-protein-3 (IGFBP-3). Best Pract Res Clin Endocrinol Metab. 2015;29(5):701–11. 10.1016/j.beem.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 17.Werner H. The IGF1 signaling pathway: from basic concepts to therapeutic opportunities. Int J Mol Sci. 2023;24(19):14882. 10.3390/ijms241914882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racine HL, Serrat MA. The actions of IGF-1 in the growth plate and its role in postnatal bone elongation. Curr Osteoporos Rep. 2020;18(3):210–27. 10.1007/s11914-020-00570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Long F. mTOR signaling in skeletal development and disease. Bone Res. 2018;6:1. 10.1038/s41413-017-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211(2):109–21. 10.1530/JOE-11-0048 [DOI] [PubMed] [Google Scholar]
- 21.Leger J, Oury JF, Noel M, Baron S, Benali K, Blot P, et al. Growth factors and intrauterine growth retardation. I. Serum growth hormone, insulin-like growth factor (IGF)-I, IGF-II, and IGF binding protein 3 levels in normally grown and growth-retarded human fetuses during the second half of gestation. Pediatr Res. 1996;40(1):94–100. 10.1203/00006450-199607000-00017 [DOI] [PubMed] [Google Scholar]
- 22.Renes JS, van Doorn J, Hokken-Koelega ACS. Current insights into the role of the growth hormone-insulin-like growth factor system in short children born small for gestational age. Horm Res Paediatr. 2019;92(1):15–27. 10.1159/000502739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motte-Signoret E, Shankar-Aguilera S, Brailly-Tabard S, Soreze Y, Dell Orto V, Ben Ammar R, et al. Small for gestational age preterm neonates exhibit defective GH/IGF1 signaling pathway. Front Pediatr. 2021;9:711400. 10.3389/fped.2021.711400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boguszewski M, Rosberg S, Albertsson-Wikland K. Spontaneous 24-hour growth hormone profiles in prepubertal small for gestational age children. J Clin Endocrinol Metab. 1995;80(9):2599–606. [DOI] [PubMed] [Google Scholar]
- 25.Boguszewski M, Rosberg S, Albertsson-Wikland K. Spontaneous 24-hour growth hormone profiles in prepubertal small for gestational age children. J Clin Endocrinol Metab. 1995;80(9):2599–606. [DOI] [PubMed] [Google Scholar]
- 26.Fattal-Valevski A, Toledano-Alhadef H, Golander A, Leitner Y, Harel S. Endocrine profile of children with intrauterine growth retardation. J Pediatr Endocrinol Metab. 2005;18(7):671–6. 10.1515/JPEM.2005.18.7.671 [DOI] [PubMed] [Google Scholar]
- 27.Rogers I, Metcalfe C, Gunnell D, Emmett P, Dunger D, Holly J. Insulin-like growth factor-I and growth in height, leg length, and trunk length between ages 5 and 10 years. J Clin Endocrinol Metab. 2006;91(7):2514–9. 10.1210/jc.2006-0388 [DOI] [PubMed] [Google Scholar]
- 28.Klammt J, Pfaffle R, Werner H, Kiess W. IGF signaling defects as causes of growth failure and IUGR. Trends Endocrinol Metab. 2008;19(6):197–205. 10.1016/j.tem.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 29.de Zegher F, Maes M, Gargosky SE, Heinrichs C, Du Caju MV, Thiry G, et al. High-dose growth hormone treatment of short children born small for gestational age. J Clin Endocrinol Metab. 1996;81(5):1887–92. [DOI] [PubMed] [Google Scholar]
- 30.Lui JC, Nilsson O, Baron J. Recent research on the growth plate: Recent insights into the regulation of the growth plate. J Mol Endocrinol. 2014;53(1):T1-9. 10.1530/JME-14-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui JC. Home for a rest: stem cell niche of the postnatal growth plate. J Endocrinol. 2020;246(1):R1-r11. 10.1530/JOE-20-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimian E, Chagin AS, Sävendahl L. Genetic regulation of the growth plate. Front Endocrinol (Lausanne). 2011;2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gafni RI, Weise M, Robrecht DT, Meyers JL, Barnes KM, De-Levi S, et al. Catch-up growth is associated with delayed senescence of the growth plate in rabbits. Pediatr Res. 2001;50(5):618–23. 10.1203/00006450-200111000-00014 [DOI] [PubMed] [Google Scholar]
- 34.van de Lagemaat M, Rotteveel J, van Weissenbruch MM, Lafeber HN. Small-for-gestational-age preterm-born infants already have lower bone mass during early infancy. Bone. 2012;51(3):441–6. 10.1016/j.bone.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 35.Deodati A, Manco M, Mariani M, Bocchini S, Högler W, Cappa M, et al. Bone density and body composition in small for gestational age children with adequate catch up growth: A preliminary retrospective case control study. Bone. 2021;153:116114. 10.1016/j.bone.2021.116114 [DOI] [PubMed] [Google Scholar]
- 36.Christmann V, van der Putten ME, Rodwell L, Steiner K, Gotthardt M, van Goudoever JB, et al. Effect of early nutritional intake on long-term growth and bone mineralization of former very low birth weight infants. Bone. 2018;108:89–97. 10.1016/j.bone.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 37.Buttazzoni C, Rosengren B, Tveit M, Landin L, Nilsson J, Karlsson M. Preterm Children Born Small for Gestational Age are at Risk for Low Adult Bone Mass. Calcif Tissue Int. 2016;98(2):105–13. 10.1007/s00223-015-0069-3 [DOI] [PubMed] [Google Scholar]
- 38.Schweizer R, Martin DD, Haase M, Roth J, Trebar B, Binder G, et al. Similar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational-age (SGA) children. Bone. 2007;41(5):875–81. 10.1016/j.bone.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 39.Smeets CCJ, van der Steen M, Renes JS, Hokken-Koelega ACS. Bone mineral density after cessation of GH treatment in young adults born SGA: A 5-year longitudinal study. J Clin Endocrinol Metab. 2017;102(9):3508–16. 10.1210/jc.2017-00269 [DOI] [PubMed] [Google Scholar]
- 40.Chou FS, Yeh HW, Chen CY, Lee GT, Parrish MR, Omede M, et al. Exposure to placental insufficiency alters postnatal growth trajectory in extremely low birth weight infants. J Dev Orig Health Dis. 2020;11(4):384–91. 10.1017/S2040174419000564 [DOI] [PubMed] [Google Scholar]
- 41.Beukers F, Rotteveel J, van Weissenbruch MM, Ganzevoort W, van Goudoever JB, van Wassenaer-Leemhuis AG. Growth throughout childhood of children born growth restricted. Arch Dis Child. 2017;102(8):735–41. 10.1136/archdischild-2016-312003 [DOI] [PubMed] [Google Scholar]
- 42.Sivakumar S, Arunprasath TS, Ramanan PV. Factors associated with catch-up growth in term, asymmetrical small-for-gestational age infants in the first year of life. Rambam Maimonides Med J. 2021;12(4):e0029. 10.5041/RMMJ.10452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vakil P, Henry A, Craig ME, Gow ML. A review of infant growth and psychomotor developmental outcomes after intrauterine exposure to preeclampsia. BMC Pediatr. 2022;22(1):513. 10.1186/s12887-022-03542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nawa N, Black MM, Araya R, Richiardi L, Surkan PJ. Pre- and post-natal maternal anxiety and early childhood weight gain. J Affect Disord. 2019;257:136–42. 10.1016/j.jad.2019.06.068 [DOI] [PubMed] [Google Scholar]
- 45.Gila-Díaz A, Carrillo GH, López de Pablo ÁL, Arribas SM, Ramiro-Cortijo D. Association between maternal postpartum depression, stress, optimism, and breastfeeding pattern in the first six months. Int J Environ Res Public Health. 2020;17(19):7153. 10.3390/ijerph17197153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321–41. 10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- 47.Ong KKL. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52(6):863–7. 10.1203/00006450-200212000-00009 [DOI] [PubMed] [Google Scholar]
- 48.Neves RO, Bernardi JR, Silva CHD, Goldani MZ, Bosa VL. Can parity influence infant feeding in the first six months of life? Cien Saude Colet. 2020;25(11):4593–600. 10.1590/1413-812320202511.01432019 [DOI] [PubMed] [Google Scholar]
- 49.Bocca-Tjeertes IF, Reijneveld SA, Kerstjens JM, de Winter AF, Bos AF. Growth in small-for-gestational-age preterm-born children from 0 to 4 years: the role of both prematurity and SGA status. Neonatology. 2013;103(4):293–9. 10.1159/000347094 [DOI] [PubMed] [Google Scholar]
- 50.Brescianini S, Giampietro S, Cotichini R, Lucchini R, De Curtis M. Genetic and environmental components of neonatal weight gain in preterm infants. Pediatrics. 2012;129(2):e455–9. 10.1542/peds.2010-0510 [DOI] [PubMed] [Google Scholar]
- 51.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46(1):4–14. 10.1111/j.1479-828X.2006.00506.x [DOI] [PubMed] [Google Scholar]
- 52.Longo S, Borghesi A, Tzialla C, Stronati M. IUGR and infections. Early Hum Dev. 2014;90(Suppl 1):S42–4. 10.1016/S0378-3782(14)70014-3 [DOI] [PubMed] [Google Scholar]
- 53.Tagarro A, Del Valle R, Dominguez-Rodríguez S, Baquero-Artigao F, Noguera-Julian A, Vives-Oñós I, et al. Growth Patterns in Children With Congenital Cytomegalovirus Infection. Pediatr Infect Dis J. 2019;38(12):1230–5. 10.1097/INF.0000000000002483 [DOI] [PubMed] [Google Scholar]
- 54.Toni L, Plachy L, Dusatkova P, Amaratunga SA, Elblova L, Sumnik Z, et al. The genetic landscape of children born small for gestational age with persistent short stature (SGA-SS). Horm Res Paediatr. 2023;97:40. 10.1159/000530521 [DOI] [PubMed] [Google Scholar]
- 55.Kozuki N, Katz J, Lee AC, Vogel JP, Silveira MF, Sania A, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. 2015;145(11):2542–50. 10.3945/jn.115.216374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cissé AH, Taine M, Tafflet M, de Lauzon-Guillain B, Clément K, Khalfallah O, et al. Cord blood leptin level and a common variant of its receptor as determinants of the BMI trajectory: The EDEN mother-child cohort. Pediatr Obes. 2022;17(11):e12955. 10.1111/ijpo.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gohlke BC, Huber A, Hecher K, Fimmers R, Bartmann P, Roth CL. Fetal insulin-like growth factor (IGF)-I, IGF-II, and ghrelin in association with birth weight and postnatal growth in monozygotic twins with discordant growth. J Clin Endocrinol Metab. 2005;90(4):2270–4. 10.1210/jc.2004-1192 [DOI] [PubMed] [Google Scholar]
- 58.Ounsted M, Sleigh G. The infant’s self-regulation of food intake and weight gain. Difference in metabolic balance after growth constraint or acceleration in utero. Lancet. 1975;1(7922):1393–7. 10.1016/S0140-6736(75)92605-7 [DOI] [PubMed] [Google Scholar]
- 59.Smith TJ. Insulin-Like Growth Factor Pathway and the Thyroid. Front Endocrinol (Lausanne). 2021;12:653627. 10.3389/fendo.2021.653627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cianfarani S, Martinez C, Maiorana A, Scirè G, Spadoni GL, Boemi S. Adiponectin levels are reduced in children born small for gestational age and are inversely related to postnatal catch-up growth. J Clin Endocrinol Metab. 2004;89(3):1346–51. 10.1210/jc.2003-031704 [DOI] [PubMed] [Google Scholar]
- 61.Cianfarani S, Maiorana A, Geremia C, Scirè G, Spadoni GL, Germani D. Blood glucose concentrations are reduced in children born small for gestational age (SGA), and thyroid-stimulating hormone levels are increased in SGA with blunted postnatal catch-up growth. J Clin Endocrinol Metab. 2003;88(6):2699–705. 10.1210/jc.2002-021882 [DOI] [PubMed] [Google Scholar]
- 62.Sovio U, Bennett AJ, Millwood IY, Molitor J, O’Reilly PF, Timpson NJ, et al. Genetic determinants of height growth assessed longitudinally from infancy to adulthood in the northern Finland birth cohort 1966. PLoS Genet. 2009;5(3):e1000409. 10.1371/journal.pgen.1000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35(2):263–85. 10.1017/S0021932003002633 [DOI] [PubMed] [Google Scholar]
- 64.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 65.Freire BL, Homma TK, Funari MFA, Lerario AM, Vasques GA, Malaquias AC, et al. Multigene sequencing analysis of children born small for gestational age with isolated short stature. J Clin Endocrinol Metab. 2019;104(6):2023–30. 10.1210/jc.2018-01971 [DOI] [PubMed] [Google Scholar]
- 66.Samsa WE, Zhou X, Zhou G. Signaling pathways regulating cartilage growth plate formation and activity. Semin Cell Dev Biol. 2017;62:3–15. 10.1016/j.semcdb.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michigami T. Regulatory mechanisms for the development of growth plate cartilage. Cell Mol Life Sci. 2013;70(22):4213–21. 10.1007/s00018-013-1346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi S, Kim HY, Cha PH, Seo SH, Lee C, Choi Y, et al. CXXC5 mediates growth plate senescence and is a target for enhancement of longitudinal bone growth. Life Sci Alliance. 2019;2(2):e201800254. 10.26508/lsa.201800254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schweizer R, Martin DD, Binder G. Increase of jump performance during GH treatment in short children born SGA. Front Endocrinol (Lausanne). 2023;14:1122287. 10.3389/fendo.2023.1122287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fazeli PK, Klibanski A. Determinants of GH resistance in malnutrition. J Endocrinol. 2014;220(3):R57-65. 10.1530/JOE-13-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Wit CC, Sas TC, Wit JM, Cutfield WS. Patterns of catch-up growth. J Pediatr. 2013;162(2):415–20. 10.1016/j.jpeds.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 72.Chagin AS, Karimian E, Sundström K, Eriksson E, Sävendahl L. Catch-up growth after dexamethasone withdrawal occurs in cultured postnatal rat metatarsal bones. J Endocrinol. 2010;204(1):21–9. 10.1677/JOE-09-0307 [DOI] [PubMed] [Google Scholar]
- 73.Won ET, Borski RJ. Endocrine regulation of compensatory growth in fish. Front Endocrinol (Lausanne). 2013;4:74. 10.3389/fendo.2013.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin IJ. Catch-up growth: basic mechanisms. Nestle Nutr Inst Workshop Ser. 2015;81:87–97. 10.1159/000365806 [DOI] [PubMed] [Google Scholar]
- 75.Huang Y, Du M, Zhuang S, Shen Z, Li Y. Impaired growth hormone receptor signaling during non-catch-up growth in rats born small for gestational age. Horm Res Paediatr. 2010;74(2):106–13. 10.1159/000313374 [DOI] [PubMed] [Google Scholar]
- 76.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487–500. 10.1038/nrg.2016.59 [DOI] [PubMed] [Google Scholar]
- 77.Abi Habib W, Brioude F, Azzi S, Salem J, Das Neves C, Personnier C, et al. 11p15 ICR1 Partial deletions associated with IGF2/H19 DMR hypomethylation and silver-russell syndrome. Hum Mutat. 2017;38(1):105–11. 10.1002/humu.23131 [DOI] [PubMed] [Google Scholar]
- 78.Schönherr N, Meyer E, Eggermann K, Ranke MB, Wollmann HA, Eggermann T. (Epi)mutations in 11p15 significantly contribute to Silver-Russell syndrome: but are they generally involved in growth retardation? Eur J Med Genet. 2006;49(5):414–8. 10.1016/j.ejmg.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 79.Bens S, Haake A, Richter J, Leohold J, Kolarova J, Vater I, et al. Frequency and characterization of DNA methylation defects in children born SGA. Eur J Hum Genet. 2013;21(8):838–43. 10.1038/ejhg.2012.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4(9):e434. 10.1038/tp.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeong HR, Han JA, Kim H, Lee HJ, Shim YS, Kang MJ, et al. Exosomal miRNA profile in small-for-gestational-age children: a potential biomarker for catch-up growth. Genes (Basel). 2022;13(6):938. 10.3390/genes13060938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Liu S, Cao L, Zhang T, Yue D, Wang L, et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget. 2017;8(49):86592–603. 10.18632/oncotarget.21246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J-X, Xie P, Li Y-S, Wen T, Yang X-C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020;70:109504. 10.1016/j.cellsig.2019.109504 [DOI] [PubMed] [Google Scholar]
- 84.Mas-Pares B, Xargay-Torrent S, Bonmati A, Lizarraga-Mollinedo E, Martinez-Calcerrada JM, Carreras-Badosa G, et al. Umbilical cord mirnas in small-for-gestational-age children and association with catch-up growth: a pilot study. J Clin Endocrinol Metab. 2019;104(11):5285–98. 10.1210/jc.2018-02346 [DOI] [PubMed] [Google Scholar]
- 85.Alderete TL, Jones RB, Shaffer JP, Holzhausen EA, Patterson WB, Kazemian E, et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes. 2021;13(1):1961203. 10.1080/19490976.2021.1961203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tadros JS, Llerena A, Sarkar A, Johnson R, Miller EM, Gray HL, et al. Postnatal growth and gut microbiota development influenced early childhood growth in preterm infants. Front Pediatr. 2022;10:850629. 10.3389/fped.2022.850629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.An J, Wang J, Guo L, Xiao Y, Lu W, Li L, et al. The impact of gut microbiome on metabolic disorders during catch-up growth in small-for-gestational-age. Front Endocrinol (Lausanne). 2021;12:630526. 10.3389/fendo.2021.630526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–7. 10.1126/science.aad8588 [DOI] [PubMed] [Google Scholar]
- 89.Schwarzer M, Gautam UK, Makki K, Lambert A, Brabec T, Joly A, et al. Microbe-mediated intestinal NOD2 stimulation improves linear growth of undernourished infant mice. Science. 2023;379(6634):826–33. 10.1126/science.ade9767 [DOI] [PubMed] [Google Scholar]
- 90.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554–63. 10.1073/pnas.1607235113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luoto R, Collado MC, Salminen S, Isolauri E. Reshaping the gut microbiota at an early age: functional impact on obesity risk? Ann Nutr Metab. 2013;63(Suppl 2):17–26. 10.1159/000354896 [DOI] [PubMed] [Google Scholar]
- 92.Jensen EA, Young JA, Mathes SC, List EO, Carroll RK, Kuhn J, et al. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: A new frontier for microbial endocrinology. Growth Horm IGF Res. 2020;53–54:101333. 10.1016/j.ghir.2020.101333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cianfarani S, Geremia C, Scott CD, Germani D. Growth, IGF system, and cortisol in children with intrauterine growth retardation: is catch-up growth affected by reprogramming of the hypothalamic-pituitary-adrenal axis? Pediatr Res. 2002;51(1):94–9. 10.1203/00006450-200201000-00017 [DOI] [PubMed] [Google Scholar]
- 94.Zhang C, Xu D, Luo H, Lu J, Liu L, Ping J, et al. Prenatal xenobiotic exposure and intrauterine hypothalamus-pituitary-adrenal axis programming alteration. Toxicology. 2014;325:74–84. 10.1016/j.tox.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 95.Rakers F, Rupprecht S, Dreiling M, Bergmeier C, Witte OW, Schwab M. Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev. 2020;117:185–97. 10.1016/j.neubiorev.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 96.Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142(5):1692–702. 10.1210/endo.142.5.8139 [DOI] [PubMed] [Google Scholar]
- 97.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16(12):3943–9. 10.1523/JNEUROSCI.16-12-03943.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Pareren Y, Mulder P, Houdijk M, Jansen M, Reeser M, Hokken-Koelega A. Adult height after long-term, continuous growth hormone (GH) treatment in short children born small for gestational age: results of a randomized, double-blind, dose-response GH trial. J Clin Endocrinol Metab. 2003;88(8):3584–90. 10.1210/jc.2002-021172 [DOI] [PubMed] [Google Scholar]
- 99.Carel JC, Chatelain P, Rochiccioli P, Chaussain JL. Improvement in adult height after growth hormone treatment in adolescents with short stature born small for gestational age: results of a randomized controlled study. J Clin Endocrinol Metab. 2003;88(4):1587–93. 10.1210/jc.2002-021123 [DOI] [PubMed] [Google Scholar]
- 100.Dahlgren J, Wikland KA. Final height in short children born small for gestational age treated with growth hormone. Pediatr Res. 2005;57(2):216–22. 10.1203/01.PDR.0000148716.71231.81 [DOI] [PubMed] [Google Scholar]
- 101.Pando R, Masarwi M, Shtaif B, Idelevich A, Monsonego-Ornan E, Shahar R, et al. Bone quality is affected by food restriction and by nutrition-induced catch-up growth. J Endocrinol. 2014;223(3):227–39. 10.1530/JOE-14-0486 [DOI] [PubMed] [Google Scholar]
- 102.Masarwi M, Gabet Y, Dolkart O, Brosh T, Shamir R, Phillip M, et al. Skeletal effect of casein and whey protein intake during catch-up growth in young male Sprague-Dawley rats. Br J Nutr. 2016;116(1):59–69. 10.1017/S0007114516001781 [DOI] [PubMed] [Google Scholar]
- 103.Bar-Maisels M, Gabet Y, Shamir R, Hiram-Bab S, Pasmanik-Chor M, Phillip M, et al. Beta palmitate improves bone length and quality during catch-up growth in young rats. Nutrients. 2017;9(7):764. 10.3390/nu9070764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bueno-Vargas P, Manzano M, Pérez-Castillo ÍM, Rueda R, López-Pedrosa JM. Dietary complex and slow digestive carbohydrates promote bone mass and improve bone microarchitecture during catch-up growth in rats. Nutrients. 2022;14(6):1303. 10.3390/nu14061303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duggan S. Vosoritide: first approval. Drugs. 2021;81(17):2057–62. 10.1007/s40265-021-01623-w [DOI] [PubMed] [Google Scholar]
- 106.Nilsson O. New treatments for achondroplasia may be efficacious in other forms of short stature. Läkartidningen. 2021;118:20204. [PubMed] [Google Scholar]
- 107.Lui JC, Colbert M, Cheung CSF, Ad M, Lee A, Zhu Z, et al. Cartilage-Targeted IGF-1 treatment to promote longitudinal bone growth. Mol Ther. 2019;27(3):673–80. 10.1016/j.ymthe.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.