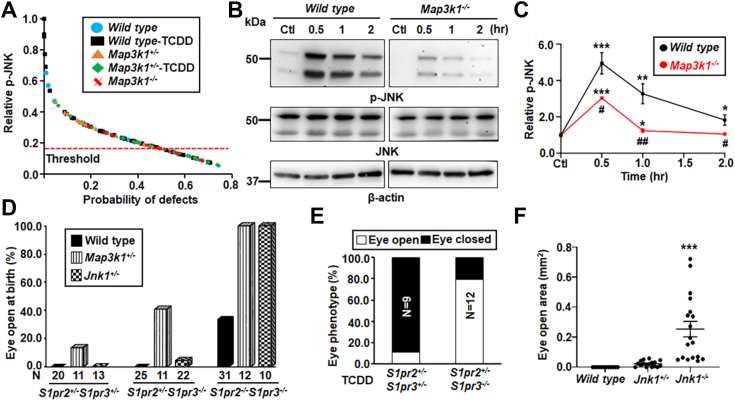
Figure 6.
Crosstalk of TCDD and the S1PR-MAP3K1-JNK1 pathway for embryonic eyelid closure.A, the levels of p-JNK in the eyelid epithelium were collected from E15.5 embryos of different genetic and exposure conditions as indicated. Data were analyzed by ImageJ software, and the relationship between the relative p-JNK level and the probability of the open-eye defects was calculated using a logistic regression model based on the binary outcome of eyelid closure, along with ROC analysis. Probability of defect was significant higher when p-JNK was below 0.17. B, The WT and Map3k1−/− keratinocytes treated with vehicle (DMSO, Ctl) or 20 μM S1P for 0.5 to 2 h and p-JNK and β-actin were examined with Western blotting. C, quantification of p-JNK using β-actin as a loading control. Data are mean ± SEM of at least three independent experiments (N ≥ 3). The S1pr2-and S1pr3-compound mutant pups under WT, Map3k1+/− and Jnk1+/− genetic backgrounds as indicated (D) without and (E) with in utero exposure to 50 μg/kg TCDD on E11.5 were collected at E17.5. The pups were examined for the eyelid open/close. N = number of pups of the indicated genotype. F, the open eye areas were measured in E17.5 WT, Jnk1+/− and Jnk1−/− fetuses exposed to TCDD (50 μg/kg body weight) at E11.5. At least 8 pups (N ≥ 8) under each condition as indicated were analyzed. Values in Ctl were set as 1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 were significantly different from Ctl of the same genotype (in C) and TCDD exposed WT (in F). #p < 0.05, ##p < 0.01 was significantly different between genotypes under the same treatment condition (in C). DMSO, dimethyl sulfoxide; JNK, Jun N-terminal kinase; MAP3K1, mitogen-activated protein kinase kinase kinase 1; ROC, receiver operating characteristic; S1P, sphigosin-1-phosphate; TCDD, 2,3,7,8-tetrachlorodibenzo-para-dioxin.