Figure 1.
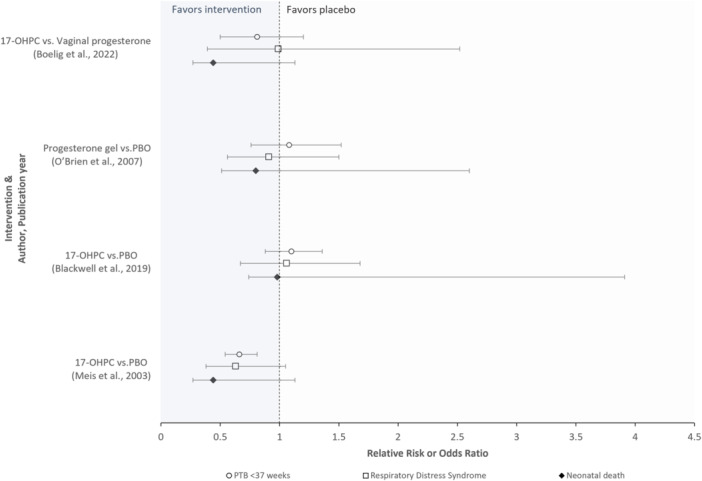
Randomized controlled trials evaluating treatment effects on the occurrence of preterm birth and, separately, neonatal morbidity/mortality outcomes (n = 4 studies). The O'Brien et al. study reported odds ratios; all other studies reported relative risks. Abbreviations: 17‐OHPC, 17‐hydroxyprogesterone caproate, PBO, Placebo, PTB, preterm birth. Interpretation: Area < 1 on the x‐axis favors the intervention, while the area > 1 favors placebo. Most trials reported no statistically significant differences between study groups across surrogate and neonatal clinical endpoints; although Meis et al. reported a statistically significant reduction attributed to the intervention for the surrogate endpoint (i.e., preterm birth), significant reductions were not reported for the neonatal clinical endpoints evaluated. The strength of surrogacy was not evaluated in any study.
