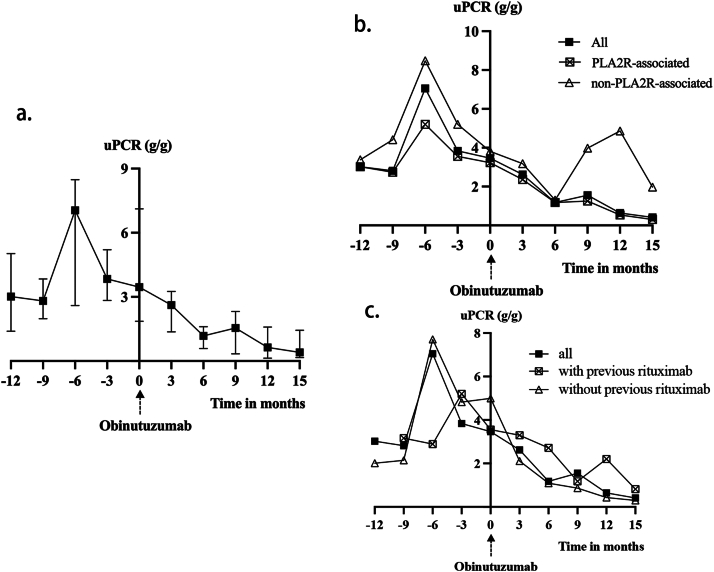
Figure 2.
(A) Change of urine protein creatinine ratio (uPCR) after obinutuzumab in all patients (n = 18). (B) Change of uPCR after obinutuzumab in PLA2R subgroups. “PLA2R-associated” (n = 15) was defined as either serum anti-PLA2R antibody titers exceeding 50 RU/mL or positive immunofluorescence staining for PLA2R antigen on kidney biopsy. (C) Change of uPCR after obinutuzumab in previous-rituximab subgroups. “With previous rituximab” (n = 12) was defined as having received at least one course of rituximab before the initial infusion of obinutuzumab.