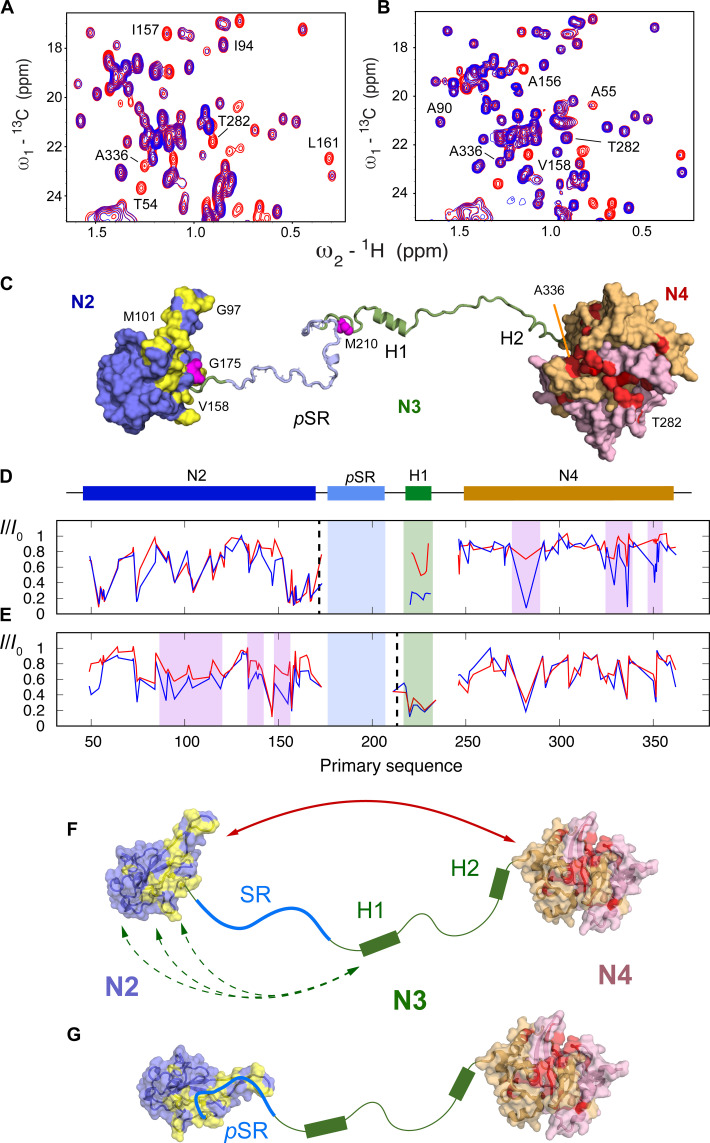
Fig. 6. Modulation of paramagnetic relaxation enhancement due to hyperphosphorylation.
(A) Comparison of 13C-1H HMQC in oxidized (blue) and reduced (red) forms of TEMPO-labeled N234 (G175C). (B) Comparison of 13C-1H HMQC in oxidized (blue) and reduced (red) forms of TEMPO-labeled pN234(II) (G175C). Strong interdomain PREs highlighted in (A) (282 and 336) are considerably weakened due to phosphorylation. (C) Surface representation indicating selected effects on long-range order in N234. Red: residues on hydrophobic surface of N4 dimer (the two monomers are otherwise colored pink and beige) that broaden in the presence of TEMPO label (175), an interaction that is weakened upon phosphorylation. Yellow: residues on N2 that shift upon hyperphosphorylation. Sites of the TEMPO labels are shown in magenta. (D and E) Intensity ratios between paramagnetic and diamagnetic forms of the protein. Blue, free N234; red, pN234(II). Dashed lines indicate position of the TEMPO label (175 and 210). (F and G) In the free, nonphosphorylated form (A), long-range interactions between N2 and N4 and between H1 and N2 are revealed from PREs. Upon hyperphosphorylation, interactions are weakened (B) or completely suppressed. CSPs on N2 (yellow) [see (C)].