ABSTRACT
This Systematic Review assesses the economic impact of Respiratory Syncytial Virus (RSV) in Latin America and the Caribbean (LAC) in relation to healthcare resource utilization and associated costs. We searched online databases from January 2012 to November 2022 to identify eligible publications. We identified 12 publications that reported direct costs, indirect costs, and resources associated with RSV and its complications. The primary direct medical resources reported were medical services, diagnostics tests and procedures, and length of stay (LOS). Direct total costs per patient ranged widely from $563 to $19,076. Direct costs are, on average, 98% higher than indirect costs. Brazil reported a higher total cost per patient than Colombia, El Salvador, México, Panamá, and Puerto Rico, while for indirect costs per patient, El Salvador and Panamá had higher costs than Brazil, Colombia, and Mexico. The mean LOS in the general ward due to RSV was 6.9 days (range 4 to 20 days) and the mean Intensive Care Unit LOS was 9.1 days (range 4 to 16 days). In many countries of the LAC region, RSV represents a considerable economic burden on health systems, but significant evidence gaps were identified in the region. More rigorous health economic studies are essential to better understand this burden and to promote effective healthcare through an informed decision-making process. Vaccination against RSV plays a critical role in mitigating this burden and should be a priority in public health strategies.
KEYWORDS: Cost of illness, direct cost, indirect cost, lower respiratory tract infections, respiratory syncytial virus
Introduction
Respiratory syncytial virus (RSV) is a prevalent seasonal virus and a leading cause of acute respiratory tract infection (ARI), including upper (URTI) and lower respiratory tract infection (LRTI). It mainly impacts infants and young children, although it can affect individuals of all age groups such as the elderly. RSV commonly leads to pneumonia and bronchiolitis. Pneumonia is an infection that inflames the lung’s air sacs, causing symptoms like coughing, fever, and difficulty breathing. Bronchiolitis, common in young children and infants, involves inflammation and congestion in the small airways (bronchioles) of the lungs. RSV is a common seasonal virus in Latin America that is a leading cause of acute respiratory tract infection.1–4 In 2019, it was estimated that there were 33 million RSV-associated acute LRTI episodes, 3.6 million related hospital admissions, and 26,300 related in-hospital deaths in children aged 0–60 months.3 RSV is also responsible for a significant economic burden worldwide including Latin America (LAC) despite probable underreporting due to lack of routine testing.5 An adequate understanding of the epidemiological and economic burden of RSV is essential to promote effective health policies in all populations including the development of prevention and therapeutic strategies.6–8 Vaccination against RSV can be a critical component of these strategies, aiming to reduce the incidence and severity of infections. Few studies systematically assessed this burden in LAC and none covered a complete age range population.6,9 We conducted a systematic review to evaluate both the epidemiological and economic burden of RSV disease burden, but the first component is reported elsewhere. We aimed to provide comprehensive estimates of RSV economic burden at all ages for LAC through a systematic review. This information aims to support informed decision-making regarding therapeutic strategies such as vaccination.
Methods
We conducted a systematic review following the Cochrane methods,10 and reported adhering to the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA)11 guidelines, as well as the Meta-analyses of Observational Epidemiology (MOOSE)12 guidelines specifically for reviews of observational studies. The study protocol is registered with the prospective international systematic review registry PROSPERO (CRD42023393731).13
Search sources and strategy
We conducted literature searches for published studies in Medline (via PubMed), Latin American and Caribbean Health Sciences Literature (LILACS), Excerpta Medica Database (EMBase), Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane Library, Center for Reviews and Dissemination (CRD) York, and EconLIT. The search strategy is detailed in the supplementary material (File S1). We did not use economic terms to limit the search because the search strategy aimed initially at finding both the epidemiological and economic burden of RSV disease. The searches were limited to articles published between January 2012 and November 2022. We searched for gray literature in generic internet and meta-search engines (Google, Google Scholar), Pan American Health Organization, Virtual Health Library, hospital reports, and Global Burden Disease (GBD) data from the Institute for Health Metrics and Evaluation (IHME).14 Additionally, relevant information was sought from the Ministries of Health in LAC countries, hospital reports, international conference proceedings, and doctoral theses.
Article selection
We included any economic design (economic evaluations and costs or budget impact studies) published or reported since 01/01/2012 assessing the economic burden of RSV in populations of all ages or basal risk status (no risk conditions/average risk or underlying medical conditions/risk factors) from LAC countries. Studies were selected independently by pairs of reviewers. Studies were excluded if they were published in languages other than Spanish, English, or Portuguese. The following types of studies were also excluded: systematic reviews, meta-analyses, narrative reviews, interventional studies, surveys not being part of a cost of illness study, non-human data, case reports, letters to the editor, newspapers, editorials, comments, opinions, molecular studies, pilot studies, protocol and pre-clinical studies, and studies lacking sufficient methodological details. Discrepancies were solved by consensus of the whole team. For the screening by title and abstract and selection by full-text phases we used COVIDENCE, an online platform used to process systematic reviews.15,16
Data extraction
The data extraction process was performed independently by pairs of reviewers and based on three predefined domains in an extraction form in a Google spreadsheet previously piloted in five random studies of those included. Firstly, we extracted the key study characteristics, including the publication type, publication year, authors, geographic location, and study design details, with a specific focus on evaluating potential biases. Secondly, information such as inclusion criteria, age, sex, sample size, latent immune-compromising conditions, and risk status for RSV. Lastly, data on diagnosis and treatment perspectives covering resource utilization for managing RSV, length of stays (LOS) in general wards and intensive care, direct costs (e.g., outpatient visits, laboratory tests, hospital LOS, medication expenses, pain and complication management, and rehabilitation), as well as indirect costs related to productivity loss, caregiver expenses, transportation, and other pertinent factors was abstracted. Discrepancies were resolved by consensus of the whole team.
Risk of bias assessment of included studies
To assess the risk of bias in economic studies, the CiCERO tool (draft version) for economic evaluations was used, since it evaluates the most relevant parameters and criteria for this type of study.17 The items evaluated include presence, absence, non-reporting, and non-applicability of criteria, such as analytical perspective, establishment of the target population, and costing methodologies. Discrepancies were solved by consensus of the whole team.
Analyses and reporting
We provided an overview of healthcare resource utilization associated with RSV. For studies that reported total direct and indirect costs per patient, adjustments were made to facilitate cross-country comparisons. Initially, the costs reported in the study were converted to the local currency of the country where the costs were reported. Whenever possible, the exchange rate reported in the original study was used; otherwise, the exchange rate reported by the World Bank was utilized for conversion to International Dollars ($) adjusted for Purchasing Power Parity (PPP).18,19 Inflation rates were derived from the information provided by the World Bank for all countries where data was available.20 All costs were adjusted to December 2022.
Results
Overview of included studies
A total of 8779 studies were identified after removing duplicates from the initial search for publications. Of these, 641 studies were selected for a full-text review based on the review eligibility criteria, 23 potentially eligible economics studies were identified for information extraction, and 12 economics studies were finally included for a descriptive analysis of the use of healthcare resources and costs (direct or indirect) associated with RSV. The details of the ten initially included and finally excluded studies due to lack of data to extract are detailed in the supplementary material (Table 1 S1). Figure 1 shows the study selection flow diagram and Table 1 presents the characteristics of the included studies.
Table 1.
Characteristics of included studies.
| Author and year of publication | Country | Study design | Population | Type of resources reported | Resources included | Perspective |
|---|---|---|---|---|---|---|
| Buendía 2021a21 | Colombia | Budget Impact Analysis | Infants (0 to 12 months of age) |
Direct and indirect | Medical consultation at emergency room, specialist referrals, chest physiotherapy, diagnosis support, medication, medical devices, day bed in the ICU, and day bed in the general medical ward | Societal |
| Buendía 2021b22 | Colombia | Cost Effectiveness Analysis | Infants (0 to 12 months of age) |
Direct and indirect | Medical consultation at emergency room, specialist referrals, chest physiotherapy, diagnosis support, medication, medical devices, day bed in the ICU, and day bed in the general medical ward | Societal |
| Buendía 2021c25 | Colombia | Cost of Illness | Infants (0 to 12 months of age) |
Direct and indirect | Medical consultation at emergency room, specialist referrals, chest physiotherapy, diagnosis support, medication, medical devices, day bed in the ICU, and day bed in the general medical ward | Societal |
| Comas-García 202028 | Mexico | Impact study | Infants (0 to 12 months of age) |
Direct | NR | Provider perspective |
| Furlan 201623 | Brazil | Cost of Illness | Adults (20 years and older) | Direct and indirect | NR | Private Sector |
| Guevara-Cuellar 201426 | Colombia | Cost Effectiveness Analysis | Preterm infants (less than 35 weeks) or with congenital heart disease | Direct | Medications, hospitalization and consultations | Third payer |
| Jara 201924 | El Salvador y Panama | Cost of Illness | Under 10 years old | Direct and indirect | Direct medical (i.e., outpatient consultation, medications, hospital fees), non-medical (transportation, child- care), and indirect costs (lost wages) | Third payer |
| Rivera-Sepulveda 201729 | Puerto Rico | Cost of Illness | Under 18 years of age | Direct | Diagnosis and hospitalizations | Private Sector |
| Rodriguez-Martinez 202030 | Colombia | Cost of Illness | Under 2 years old | Direct | Medical and therapy services, diagnostics tests and procedures, consumables, and hospital stay | Provider perspective |
| Rodríguez‐Martínez 202231 | Colombia | Cost Effectiveness Analysis | Under 6 years old | Direct | NR | Provider perspective |
| Cintra 201327 | Brazil | Cost Effectiveness Analysis | Under 2 years old with congenital heart disease | Direct | Hospitalization in intensive care, hospitalization for treatment of pneumonia and drug (Palivizumab) | Private Sector |
| Da Silva 202232 | Brazil | Cost of Illness | Under 2 years old | Direct | NR | Private Sector |
NR, not reported.
Figure 1.
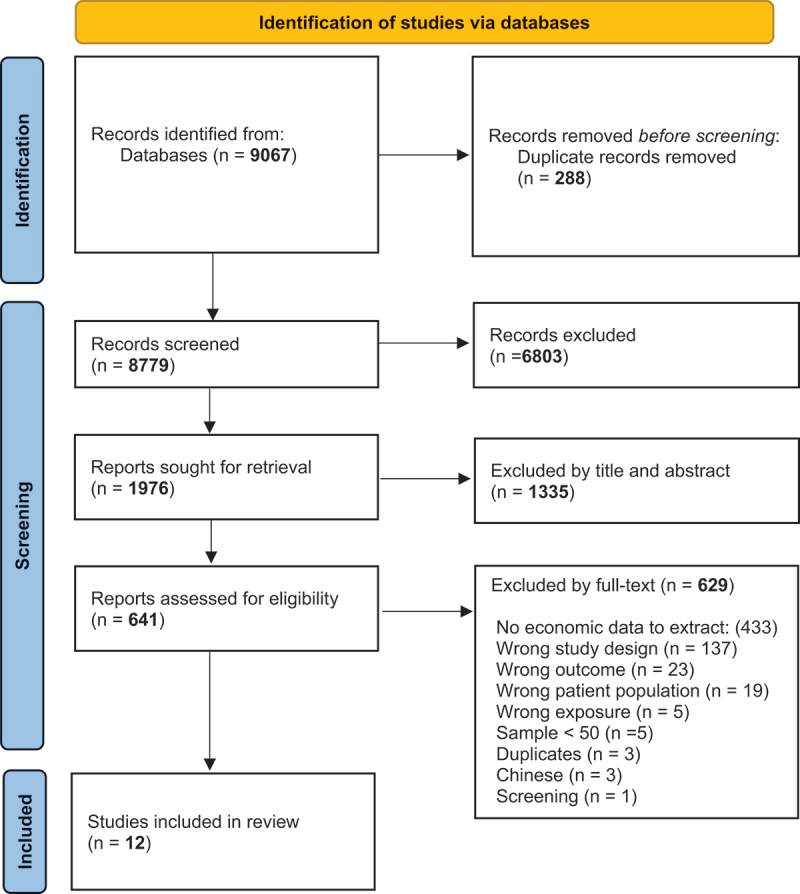
PRISMA study flow diagram.
The 12 included studies reported data from Colombia (n = 6), Brazil (n = 3), Mexico (n = 1), Puerto Rico (n = 1), and one from Salvador and Panama (n = 1). Most of the included studies reported costs of disease (n = 7), followed by cost-effectiveness evaluations (n = 4), budget Impact Analysis (n = 1), and impact studies (n = 1). While all studies provide data on direct medical costs, only five reported indirect costs of RSV.21–25 All studies, except one, were conducted in the pediatric population. Two of these studies26,27 were specifically conducted in pediatric populations with congenital heart disease. The perspective of the studies was societal (n = 3), from the provider (n = 3), from the third payer (n = 2), from the public sector (n = 2) and the private sector (n = 2).
Health care resource use
Table 2 shows a summary of both direct and indirect expenses. Across all studies, direct resource utilization encompassed doctor consultations, medication, hospital stays, nursing care, and prescriptions. Indirect resource utilization encompassed the patient’s and family member’s lost workdays, reduced work productivity, and the necessity for caregiving. The costs are presented as costs per day of hospitalization in the general ward and ICU, the total and average cost per case in the general ward and ICU, and the indirect cost per casea.
Table 2.
Costs of included studies in USD PPA 2022.
| Author and year of publication | Country | Cost per ICU hospitalization day | Cost per hospital day General Ward | Total cost per case in ICU | Total cost per case in general ward | Average total cost | Indirect cost per case |
|---|---|---|---|---|---|---|---|
| Buendía 2021a21 | Colombia | $847.76 | $126.43 | $976.14 | $254.81 | $1,142.09 | $40.66 |
| Buendía 2021b22 | Colombia | $788.19 | $117.55 | $907.54 | $236.90 | $1,061.83 | $19.63 |
| Buendía 2021c25 | Colombia | NR | $57.61 | NR | $213.84 | NR | $36.62 |
| Comas-García 202028 | Mexico | $662.88 | NR | $1,367.53 | NR | NR | NR |
| Furlan 201623 | Brazil | NR | NR | NR | NR | $19,075.90 | $64.13 |
| Guevara-Cuellar 201426 | Colombia | $733.24 | $118.52 | NR | NR | NR | NR |
| Jara 201924 | El Salvador | $274.93 | NR | NR | NR | $562.68 | $130.22 |
| Panamá | $693.43 | NR | NR | NR | $693.43 | $169.55 | |
| Rivera-Sepulveda 201729 | Puerto Rico | NR | NR | NR | NR | $5,168.25 | NR |
| Rodriguez-Martinez 202030 | Colombia | $810.21 | $87.01 | NR | NR | NR | NR |
| Rodríguez‐Martínez 202231 | Colombia | NR | NR | NR | NR | $1,537.85 | NR |
| Cintra 201327 | Brazil | $673.53 | NR | NR | NR | $10,776.49 | NR |
| Da Silva 202232 | Brazil | NR | NR | $8,029.46 | $1,889.92NR | $2,805.04 | NR |
NR: not reported.
Buendía et al.21,22,25 reported an average LOS of 5.8 days in general wards and 10 days in ICU. Comas-García28 reported an average ICU LOS of 10 days. Jara24 reported an average LOS of 4 days in general wards and 5 in the ICU, Rodríguez-Martínez31 4.4 and 10.2 days, respectively, and Cintra27 20 and 16 days, respectively. Notably, the study by Cintra,27 which reported higher lengths of stay, was conducted specifically in a pediatric population with congenital heart disease, which may explain the longer durations in both general wards and ICU.
Risk of bias among included studies
The evaluation of the quality of the four included cost-effectiveness analysis with the CiCERO tool18 is provided in Table 3. The majority of these economic analyses demonstrated satisfactory quality. However, one study omitted the use of a discount rate, and none of the studies adjusted their cost estimates for changes in inflation rates. Only one study included indirect costs. One study reported conflicts of interest and the remaining did not provide statements regarding conflict of interest.
Table 3.
Quality evaluation for cost-effectiveness analysis.
| Quality Assessment (CiCERO) | Buendía 2021b22 | Guevara-Cuellar 201426 | Rodríguez‐Martínez 202231 | Cintra 201327 |
|---|---|---|---|---|
| Country of conduct | Yes | Yes | Yes | Yes |
| Population of analysis | Yes | Yes | Yes | Yes |
| Time horizon | Yes | Yes | Yes | Yes |
| Perspective | Yes | Yes | Yes | Yes |
| Discount Rate | Yes | Yes | NA | No |
| Adjustment of inflation | No | No | NA | No |
| Interventions compared | Yes | Yes | Yes | Yes |
| Direct Costs, Capital Costs | Yes | Yes | Yes | Yes |
| Indirect Medical Costs | No | No | No | No |
| Indirect Costs | Yes | No | No | No |
| Valuation of Effectiveness? | Yes | Yes | Yes | Yes |
| Compliance/adherence with treatment | No | No | No | No |
| Decision Modelling? | Yes | Yes | Yes | Yes |
| Cost/Health and/or Economic Outcomes? | Yes | Yes | Yes | No |
| Uncertainty | Yes | Yes | Yes | Yes* |
| Conflicts of interest and sources of funding | No | Yes | No | NR |
| Software | Yes | Yes | Yes | Yes |
| Were the cost data standardized? | Yes | Yes | Yes | Yes |
| Was the data synthesised in a de-aggregated manner | Yes | No | Yes | No |
| Synthesis appropriate to the target audience? | Yes | Yes | Yes | Yes |
| Separation between “RCT-based economic evaluations” and modeling studies results? | NA | NA | NA | NA |
| DSA and PSA reported separately? | Yes* | Yes* | Yes | Yes* |
NA, not applicable; NR, not reported; DSA, deterministic sensitivity analysis; PSA, probabilistic sensitivity analysis. *: Reporting only deterministic sensitivity analysis.
Country-specific burden data from ministries of health’s databases
Country-specific burden data are only available from Brazil since these are not accessible from Colombia. Health information from the Information Department of the Unified Health System (DATASUS for its acronym in Portuguese) was used.33 The data related to hospital morbidity, which are published by DATASUS, have their origin in the SUS Hospital Information System (SIH/SUS). The hospital units associated with the SUS transmitted their admission records to the municipal or state authorities through the Hospitalization Authorization (AIH). Total costs for bronchiolitis, which encompass hospital costs and professional services costs, are available in the hospital morbidity database. These costs are defined based on the AIH approved during the period considered as approved production cost. From these data, information was collected on costs (total cost, hospital costs, and professional services costs) and resource use (number of days and average hospital stay).
Table 4 presents the number of hospitalizations for bronchiolitis, number of days of hospitalization per patient, and cost per day of hospitalization (in dollars) extracted from the DATASUS database presented annually from 2012 to 2022. The exchange rate from dollar to real is also presented for each year of analysis.
Table 4.
Costs of hospitalizations for bronchiolitis in the public health system (SUS) 2012–2022. Values in international dollars ($) per year.
| Year | Hospitalizations (N) | Length of stay | Unit cost per day of hospitalization in USD | Exchange rate BRL to USD |
|---|---|---|---|---|
| 2012 | 54,115 | 4.2 | $41.37 | R$1.95 |
| 2013 | 57,284 | 4.2 | $36.34 | R$2.16 |
| 2014 | 51,522 | 4.2 | $35.92 | R$2.35 |
| 2015 | 55,760 | 4.2 | $26.91 | R$3.33 |
| 2016 | 53,315 | 4.3 | $25.36 | R$3.49 |
| 2017 | 61,555 | 4.3 | $29.31 | R$3.19 |
| 2018 | 65,682 | 4.3 | $27.40 | R$3.65 |
| 2019 | 67,638 | 4.3 | $26.64 | R$3.94 |
| 2020 | 22,168 | 4.0 | $24.20 | R$5.16 |
| 2021 | 46,785 | 4.2 | $25.73 | R$5.39 |
| 2022 | 81,041 | 4.4 | $27.30 | R$5.16 |
Source: Brazilian Ministry of Health.
In the database, a total cost for medical care is recorded that amounted to approximately US$9.7 dollars in 2022. In addition, the average cost of hospitalization for bronchiolitis for the same year was approximately US$120.
Discussion
We conducted a systematic review describing the economic burden of RSV in terms of healthcare resource use and costs in LAC from January 2012 to November 2022. We included 12 publications reporting direct costs, indirect costs, and resources associated with RSV and its complications. The minimum general ward LOS for RSV was 4 days, the maximum of 20 days, and the average of 6.9 days, and 4, 16, and 9.1 days in ICU, respectively. In general, the results of LOS show similar ranges between the studies analyzed, except in the case of Cintra et al.27 the study is limited to infants with congenital heart diseases. While most studies report an average duration of between 4 and 10 days, Cintra et al.27 reports a considerably longer average LOS, with an average of 20 days in general wards and 16 days in ICU. This discrepancy could be attributed to the complexities associated with the clinical management of this specific population.
The main direct medical costs reported in the studies included medical services, diagnostic tests, procedures, and hospital stays. Notably, significant differences in average total direct costs per patient were observed among studies conducted in Brazil (Furlan23 $19,076, Cintra27 $10,776, and da Silva32 $2,805), reflecting variations in the studied populations. Furlan focused on adults, Cintra on children under 2 years with congenital heart disease, and da Silva on children under 2 years in general. In the context of congenital heart disease, it is essential to highlight Guevara-Cuellar’s study, which specifically examines children under two years old with this condition and emphasizes the necessity of passive immunization with palivizumab. Total direct costs per patient ranged from $563 to $10,015, with Brazil exhibiting the highest costs. On average, direct costs exceeded indirect costs by 98%.
The economic impact linked to respiratory syncytial virus (RSV), as per the findings of this systematic review, is substantial, even when juxtaposed with other respiratory ailments. For instance, a study conducted by Pineda-García34 revealed that the average annual cost per hospitalized patient with severe asthma in Colombia amounted to USD $1,016 (adjusted for inflation up to 2022 and for PPP). In contrast, the costs per hospitalization attributable to RSV span between USD $1,061 and USD $1,537, as indicated by the studies scrutinized in this review. Moreover, the research conducted by Pascal Crépey et al.35 indicates that the average expense per hospitalization due to influenza in Brazil’s private sector stands at USD $919.52 (adjusted for inflation to 2022 and for PPP). By comparison, the expenses associated with RSV in the same sector range from USD $10,015 to USD $19,075 per hospitalization.
When we examined the database of the DATASUS of Brazil, heterogeneity in costs is observed compared to the studies analyzed. This may be explained by the fact that the studies were carried out in private hospitals, while the DATASUS data come from the public health sector. These findings suggest the substantial economic burden of RSV in LAC, confirming our results of a previous systematic review in the LAC region.9 Other studies arrived at similar conclusions. Our results also highlight disparities in direct medical costs between countries in the region, which can be attributed to a variety of factors, such as differences in health infrastructure, access to specialized medical services, availability of advanced medical technology, levels of development, economics and medical care financing systems. In addition, variations in health policies, prices of medical supplies, and labor costs may also influence these differences.36
Grace et al.,5 conducted a systematic review of the global economic burden and use of healthcare resources in older adults affected by RSV. They analyzed 42 studies that provided cost and resource utilization data on adult patients worldwide, without language or geographic location restrictions. The results highlighted that hospitalization costs were higher in the United States (US), attributing this increase to factors such as older age, more comorbidities, and higher LOS. Despite limitations, such as variability in cost definitions, age groups, geographic locations, and the lack of data outside the United States, the study concluded that the economic burden of RSV worldwide was considerable. Another systematic review37 conducted for the United States aimed to examine the costs associated with children affected by respiratory syncytial virus (RSV). Key findings revealed variability in mean annual hospital costs per RSV patient, ranging from $9,825 for full-term infants to $26,120 for late preterm infants in 2020.Compared to our study, it makes sense that the cost ranges would be higher in the US than those presented in our review, which includes only studies from LAC.
Ramos et al.38 analyzed the costs of treating hospitalized pediatric patients with RSV and Parainfluenza Virus Type 3 (PIV3) in upper-middle-income countries through a systematic review through July 2022. Cost analyses were reported for Colombia, China, Malaysia, and Mexico. Costs varied widely, being lowest in Malaysia and highest in Colombia per patient in the pediatric ward, while in the pediatric ICU, the lowest costs were in China and the highest in Mexico. In summary, the study also underscores the significant economic burden of RSV for health systems and highlights the potential relationship between patients’ baseline risk and expenditures, as well as the frequent prescription of treatments without support in clinical practice guidelines.
The majority of acute lower respiratory infection cases linked to RSV, exceeding 95%, as well as over 97% of RSV-related deaths across all age groups, occurred in low-income and middle-income countries (LMICs), including many nations in LAC.3 A systematic literature review that included 17 studies published between 2014, and 2021, assessed the RSV-related costs in US children aged 0–59 months. Full-term infants accounted for 82% of RSV hospitalizations (RSVHs) and 70% of RSVH costs, with RSVHs of extremely premature infants costing 5.6 times more. Infant RSV treatment costs 2020 USD$187 per overall birth and $227 per publicly funded birth.37
Our study has strengths such as the use of a sound methodology focused on populations of all ages from a specific region with limited access to data like LAC, and taking advantage of our previous research on the same topic.9 By using 2022 international dollars adjusted for PPP and inflation rates, our cost estimates can be easily compared with the estimates of other studies. Additionally, we performed the quality assessment of the included cost-effectiveness analysis with the CiCERO tool17 considered the most comprehensive available instrument. Nevertheless, our study has several limitations. We couldn’t access the Colombian Ministry of Health databases and we cannot discard potentially relevant information from other countries without public online access. There was scarce information about non hospitalized episodes. The wide variability of cost found could be due to heterogeneity in cost definitions, age groups, and health systems. Also RSV is not the only cause of bronchiolitis, so some heterogeneity could arise from that fact. We found substantial heterogeneity of reported studies, and this limits comparison and averaging them, and not always power parity adjustment suffices to allow for averaging results. Finally, underreporting of RSV cases by surveillance systems may lead to an understatement of the real economic burden.
Adequately understanding the epidemiological burden of the diseases of interest and their impact on the health system is essential to define effective healthcare policies for all populations.6 Considering the economic burden of RSV may lead to the formulation of policies that not only reduce associated costs but also improve the quality of life of affected communities.8 A deeper understanding of the economic burden of RSV empowers health policymakers to make more informed decisions,39 involving a more efficient allocation of resources, the development of prevention, including vaccination, and health promotion strategies aimed at vulnerable populations, and ensuring the availability of appropriate treatments and care.5,7 Our findings underline the urgency of implementing preventive strategies to reduce the significant economic impact of RSVin LAC and provide valuable insights to inform healthcare planning and health policy.
Conclusion
This systematic review shows that although evidence is limited, RSV and its sequelae impose a substantial economic burden on many countries of LAC, but significant evidence gaps were identified for most countries of the region. The results support the need for preventative strategies such as vaccination and improved disease management to minimize the RSV-associated economic disease burden in the LAC region.
Supplementary Material
Funding Statement
The study was supported by an independent grant of Pfizer’s Centers for Therapeutic Innovation.
Note
All costs presented in Table 2 are data provided by the studies with inflation adjustment to 2022 for all countries and with change by PPP(see methods section).
Disclosure statement
JL and RSdA are employees of Pfizer Inc and may hold stock or stock options.
The other authors have no conflicts of interest to declare.
Author’s contributions
AC, AB, CM, JL, and RSdA participated in the conception/design of the review; AB, AC, CM, CP, MS, SR, KS, and SAR participated in the collection/assembling of the data; AC, AB, CM, JL, RUG and RSdA, EB, SR, CP, MS, SR, KS, and SAR performed/supervised the analysis; AC, AB, JL, and RSdA, EB, SR and RUG participated to the interpretation of the data in the application of the methodology. All authors agreed to the publication of the present work.
Data availability statement
All relevant data are within the manuscript.
Notes on contributor
We are researchers from the Health Technology Assessment (HTA) and Health Economics Department within the Institute for Clinical Effectiveness and Health Policy (IECS). Our department is affiliated with Cochrane Argentina and is part of the Cochrane Collaboration and the Cochrane Iberoamerican Network (RCIB). In collaboration with the Rosario Perinatal Studies Center (CREP) and the Hospital Italiano University Institute (IUHI), we lead Cochrane Argentina. Our academic efforts focus on conducting systematic literature reviews, meta-analyses, and methodological research related to evidence-based medicine. Our mission is to facilitate clinical and health decision-making based on the best available scientific evidence. We are committed to developing knowledge and tools that guide the establishment of more effective, efficient, and equitable health systems in Latin America and other low- and middle-income countries.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2381298
References
- 1.CDC . Learn about respiratory syncytial virus infection (RSV). Centers for Disease Control and Prevention [Internet]. 2024. Jan 5 [accessed 2024 Mar 22]. https://www.cdc.gov/rsv/index.html.
- 2.Bronquiolitis . Argentina.gob.ar [Internet]. 2017. Aug 16 [accessed 2024 Mar 22]. https://www.argentina.gob.ar/salud/glosario/bronquiolitis.
- 3.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EA, Campbell H.. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022; 399. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suryadevara M, Domachowske JB. Epidemiology and Seasonality of Childhood Respiratory Syncytial Virus Infections in the Tropics. Viruses. 2021;13(4):696. doi: 10.3390/v13040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace M, Colosia A, Wolowacz S, Panozzo C, Ghaswalla P. Economic burden of respiratory syncytial virus infection in adults: a systematic literature review. J Med Econ. 2023;26(1):742–8. doi: 10.1080/13696998.2023.2213125. [DOI] [PubMed] [Google Scholar]
- 6.Mosadeghrad AM, Jaafaripooyan E, Zamandi M. Economic Evaluation of Health Interventions: A Critical Review. Iran J Public Health. 2022;51:2159. doi: 10.18502/ijph.v51i10.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Economic Evaluation Overview . 2021. Mar 16 [accessed 2024 Mar 22]. https://www.cdc.gov/policy/polaris/economics/index.html#:~:text=Public%20health%20professionals%20can%20use,of%20different%20public%20health%20interventions.
- 8.Bal R, Wallenburg I. Linking Costs and Quality in Healthcare: towards Sustainable Healthcare Systems; Comment on “Hospitals Bending the Cost Curve With Increased Quality: A Scoping Review Into Integrated Hospital Strategies. Int J Health Policy Manag. 2023;12:1–3. doi: 10.34172/ijhpm.2022.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardach A, Rey-Ares L, Cafferata ML, Cormick G, Romano M, Ruvinsky S, Savy V. Systematic review and meta-analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. 2014;24(2):76–89. doi: 10.1002/rmv.1775. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane Handbook for Systematic Reviews of Interventions . [accessed 2024 Mar 22]. https://training.cochrane.org/handbook/current.
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Ciapponi A, Bardach A, Comande D, Carolina Palermo M, Macarena Sandoval M, Stegelmann K, Ardiles S, Ruvinsky S, Baumeister E. Burden of respiratory syncytial virus disease in Latin America: A protocol for a systematic review and meta-analysis. PROSPERO International prospective register of systematic reviews [Internet]. [accessed 2024 Mar 22]. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=393731.
- 14.GBD Compare . Institute for Health Metrics and Evaluation [Internet]. [accessed 2024 Mar 22]. http://vizhub.healthdata.org/gbd-compare.
- 15.View of Product Review: Covidence (Systematic Review Software) . [accessed 2024 Mar 22]. https://journals.library.ualberta.ca/jchla/index.php/jchla/article/view/22892/17064.
- 16.Covidence - Better systematic review management . Covidence [Internet]. 2020. June 11 [accessed 2024 Mar 22]. https://www.covidence.org/.
- 17.Mandrik OL, Severens JLH, Bardach A, Ghabri S, Hamel C, Mathes T, Vale L, Wisløff T, Goldhaber-Fiebert JD. Critical Appraisal of Systematic Reviews With Costs and Cost-Effectiveness Outcomes: An ISPOR Good Practices Task Force Report. Value Health. 2021;24(4):463–472. doi: 10.1016/j.jval.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Official exchange rate (LCU per US$, period average) . World Bank [Internet]. https://data.worldbank.org/indicator/PA.NUS.FCRF.
- 19.PPP conversion factor, GDP (LCU per international $) . World Bank [Internet]. https://data.worldbank.org/indicator/PA.NUS.PPP.
- 20.Inflation, consumer prices (annual %) . World Bank Open Data [Internet]. [accessed 2024 Mar 22]. https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG.
- 21.Buendía JA, Acuña-Cordero R, Rodriguez-Martinez CE. Budget impact analysis of high-flow nasal cannula for infant bronchiolitis: the Colombian National Health System perspective. Curr Med Res Opin. 2021;37(9):1627–1632. doi: 10.1080/03007995.2021.1943342. [DOI] [PubMed] [Google Scholar]
- 22.Buendía JA, Acuña-Cordero R, Rodriguez-Martinez CE. The cost-utility of early use of high-flow nasal cannula in bronchiolitis. Health Econ Rev. 2021;11(1):1–8. doi: 10.1186/s13561-021-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlan TM. Estudo da viabilidade para introduzir na rotina testes de diagnóstico para infecção respiratória aguda. Universidade de São Paulo; 2016. doi: 10.11606/T.5.2016.tde-30062016-161948. [DOI] [Google Scholar]
- 24.Jara JH, Azziz-Baumgartner E, De Leon T, Luciani K, Brizuela YS, Estripeaut D, Castillo JM, Barahona A, Corro M, Cazares R, et al. Costs associated with acute respiratory illness and select virus infections in hospitalized children, El Salvador and Panama, 2012–2013. J Infect. 2019;79(2):108–114. doi: 10.1016/j.jinf.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Buendía JA, Patiño DG. Costs of Respiratory Syncytial Virus Hospitalizations in Colombia. Pharmacoecon Open. 2021;5(1):71–6. doi: 10.1007/s41669-020-00218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guevara-Cuéllar CA. Costo utilidad de profilaxis con palivizumab versus no profilaxis en niños con riesgo de infección de virus sincitial respiratorio en Colombia. CES med. 2014;28(2):203–219. [Google Scholar]
- 27.Cintra MADCT. Avaliação do custo efetividade da profilaxia do vírus sincicial respiratório em lactentes com cardiopatia congênita. 2013. https://www.arca.fiocruz.br/handle/icict/9313.
- 28.Comas-García A, Aguilera-Martínez JI, Escalante-Padrón FJ, Lima-Rogel V, Gutierrez-Mendoza LM, Noyola DE. Clinical impact and direct costs of nosocomial respiratory syncytial virus infections in the neonatal intensive care unit. Am J Infect Control. 2020;48(9):982–986. doi: 10.1016/j.ajic.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Rivera-Sepulveda A, Garcia-Rivera EJ. Epidemiology of bronchiolitis: a description of emergency department visits and hospitalizations in Puerto Rico, 2010–2014. Trop Med Health. 2017;45(1):1–0. doi: 10.1186/s41182-017-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Direct medical costs of RSV-related bronchiolitis hospitalizations in a middle-income tropical country. Allergol Immunopathol. 2020;48(1):56–61. doi: 10.1016/j.aller.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Martínez CE, Sossa-Briceño MP, Nino G. Emergency department-initiated home oxygen for viral bronchiolitis: A cost-effectiveness analysis. Pediatr Pulmonol. 2022;57(9):2154–2160. doi: 10.1002/ppul.26018. [DOI] [PubMed] [Google Scholar]
- 32.da Silva GT, Moreira R, Paiva AP, Regis C. Use of Health Resources and Micro-Costing Analysis of Hospitalization for Bronchiolitis and Pneumonia in Pediatric Patients: A Retrospective Study From Brazilian Perspective. doi: 10.1016/j.jval.2022.09.717. [DOI] [Google Scholar]
- 33.DATASUS – Ministério da Saúde . [accessed 2024 Mar 22]. https://datasus.saude.gov.br/.
- 34.García YRP. Costos médicos directos de la atención del asma en personas menores de 18 años en el servicio de urgencias Bogotá en el año 2019. Biblioteca digital UdeA [Internet]. https://www.google.com/url?q=https://bibliotecadigital.udea.edu.co/bitstream/10495/33341/1/PinedaYaneth_2022_CostosMedicosAsma.pdf&sa=D&source=docs&ust=1712259482657878&usg=AOvVaw0mFmjiEGLMh6cNZV2yxF_9.
- 35.Crépey P, Boiron L, Araujo RR, Lopez JG, Petitjean A, de Albuquerque Luna EJ. Impact of quadrivalent influenza vaccines in Brazil: a cost-effectiveness analysis using an influenza transmission model. BMC Public Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-09409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.el Caribe CEPAL y . Salud y desigualdad en América Latina y el Caribe: la centralidad de la salud para el desarrollo social inclusivo y sostenible. 2023.
- 37.Bowser DM, Rowlands KR, Hariharan D, Gervasio RM, Buckley L, Halasa-Rappel Y, Glaser EL, Nelson CB, Shepard DS. Cost of Respiratory Syncytial Virus Infections in US Infants: Systematic Literature Review and Analysis. J Infect Dis. 2022;226(Supplement_2):225–35. doi: 10.1093/infdis/jiac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocha-Filho CR, Ramalho GS, Martins JWL, Lucchetta RC, Pinto ACPN, da Rocha AP, Trevisani GFM, Reis FSDA, Ferla LJ, Mastroianni PDC, et al. Economic burden of respiratory syncytial and parainfluenza viruses in children of upper-middle-income countries: a systematic review. J Pediatr. 2023;99(6):537–545. doi: 10.1016/j.jped.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer G, Bossi L, Santoalla M, Rodríguez S, Fariñaa D, Speranz AM. Impacto de un programa de prevención de infecciones respiratorias en lactantes prematuros de alto riesgo: estudio prospectivo y multicéntrico. [accessed 2024 Mar 22]. https://www.sap.org.ar/docs/archivos/2009/arch09_2/v107n2a04.pdf. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript.


