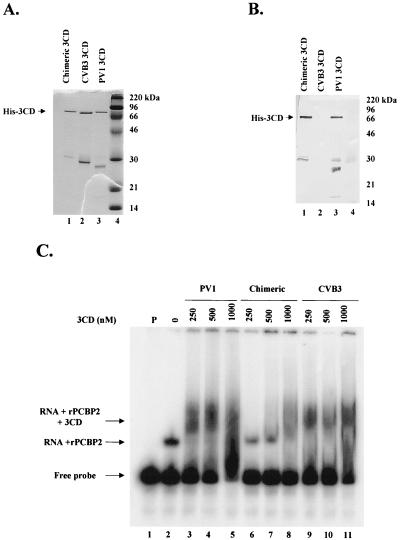
FIG. 4.
Purification and biochemical analysis of His-tagged 3CD proteins expressed in E. coli. His-tagged proteins were expressed in BL21(DE3) cells and affinity purified. (A) Coomassie blue staining of a 12.5% polyacrylamide–SDS gel following electrophoresis of fractions (lane 1, chimeric 3CD; lane 2, CVB3 3CD; lane 3, PV1 3CD) purified on a nickel column. Lane 4 contains marker proteins. (B) Western blot analysis of a gel identical to that shown in panel A. Antiserum raised against PV1 3C was used for immunoblot analysis. (C) RNA electrophoretic mobility shift analysis with an RNA probe representing the PV1 5′ CL structure. PV1 probe was incubated with buffer alone (lane 1), with recombinant PCBP2 alone, or with recombinant PCBP2 and the indicated amounts of 3CD (lanes 3 to 11). RNA-protein complexes were resolved on a 4% native polyacrylamide gel and are indicated to the left of the autoradiograph.