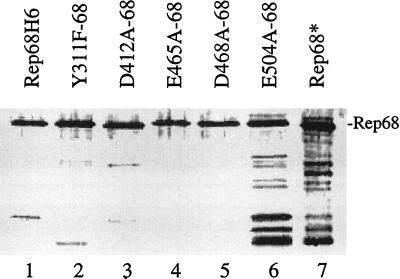
FIG. 4.
SDS-polyacrylamide gel electrophoresis–silver stain analysis of purified mutant and wt Rep68H6 proteins overexpressed in E. coli. Proteins were expressed from the inducible expression cassette pStump68 and purified via a His6 tag by passage over nickel-agarose columns (Qiagen) as described in Materials and Methods. The position of full-length Rep68H6 is indicated. The same amount of protein was loaded in each lane, except for E504A-68, which required 50-fold more protein to achieve the same level of full-length protein. The lower-molecular-weight products visible in the gel (especially in lanes 6 and 7) were mostly degradation products as indicated by Western blot analysis (data not shown). Rep68* was expressed in a baculovirus system and purified as described previously (30).