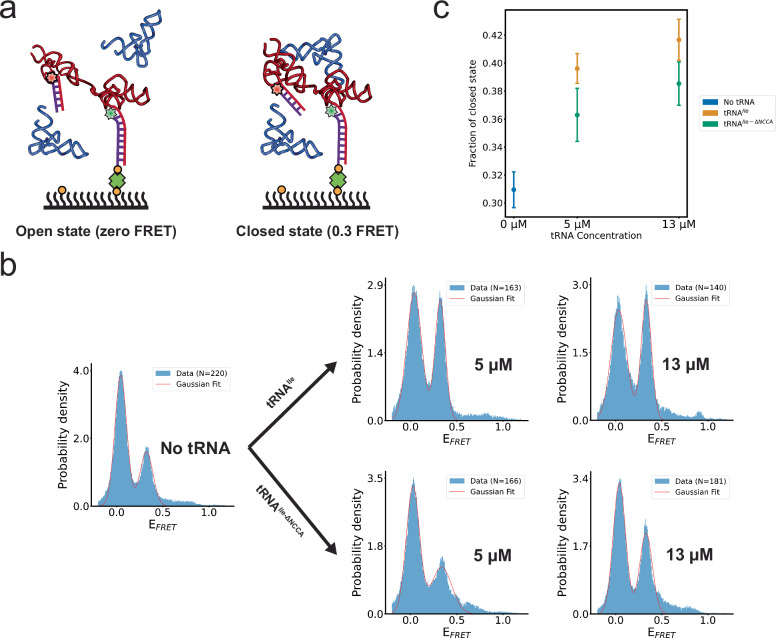
Fig. 5. The Mtb-ileS T-box riboswitch samples open and closed conformations.
a Schematic diagram illustrating the smFRET experiment using an intramolecularly labeled Mtb-ileS T-box construct. Two FRET states were observed, assigned to an open (zero FRET, left) and a closed (0.3 FRET, right) state. Cartoon based on a similar cartoon from19. b FRET efficiency histograms of intramolecularly labeled Mtb-ileS T-box in the absence of tRNA (left), and in the presence of different concentrations of tRNAIle (right, top) and tRNAIle-ΔNCCA (right, bottom). The orange line corresponds to the fits of a Gaussian function to each peak. N reports the number of traces in each histogram. Only the first 40 frames from FRET trajectories are included in the histogram to avoid including data after photobleaching of the fluorophores. c Changes in the fraction of the closed conformation as a function of tRNAIle or tRNAIle-ΔNCCA concentration. The fraction of the closed state was calculated by 1) fitting Gaussian curves to each peak, which provided an estimate of the standard deviation, 2) using these fits to obtain the area under each peak by integration, and 3) calculating the fraction by dividing the area corresponding to the 0.3 FRET state by the sum of the areas corresponding to the zero and the 0.3 FRET states in (b). The error of each fraction was estimated by standard error propagation using the fitting uncertainties as described in the Methods section and correspond to the error bars in the figure. Source data are provided as a Source Data file.