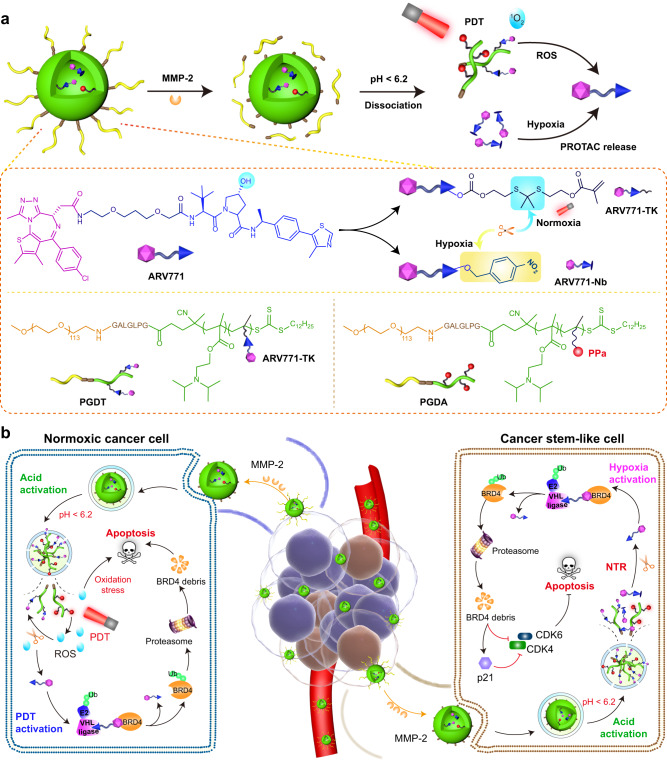
Fig. 1. Schematic illustration of the region-confined PROTAC nanoplatform for spatiotemporally tunable protein degradation and combinatory cancer therapy.
a Structure of the ROS/hypoxia dual-activatable PROTAC nanoparticle (PGDAT@N) and its responsive process to MMP-2 enzyme, intracellular acidic microenvironment, and PDT-based ROS or CSCs-relied hypoxia. b Cartoon illustration of the PGDAT@N nanoparticle eliminates tumor cells in normoxic and hypoxic areas simultaneously by self-complementary degrading BRD4 protein. PGDAT@N nanoparticle reaches tumor tissue after i.v. injection through EPR effect firstly, and MMP-2-induced PEG-deshielding enhances its accumulation and penetration at the tumor site. After internalized into tumor cells, PROTAC nanoparticle recovers its photoactivity due to acid-liable DPA groups caused dissociation of nanoparticle, and then affluent ROS in the normoxic region is generated under laser irradiation to release original PROTAC via cleaving the TK linkage. The BRD4 removal and PDT synergistically induce apoptosis of the normoxic tumor cells. Meanwhile, upon internalization by CSCs, hypoxia-activatable PROTAC derivate is divulged from the dissociated PGDAT@N nanoparticle, and then restored to parental PROTAC with nitroreductase (NTR) for sweeping tumor cells. BRD4 degradation can downregulate cell cycle proteins including cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase 6 (CDK6), meanwhile upregulating cyclin-dependent kinase inhibitor 1 A (p21) to induce apoptosis of CSCs. The obliteration of both normoxic and hypoxic tumor cells with the region-confined PROTAC nanoplatform enable tumor regression efficiently.