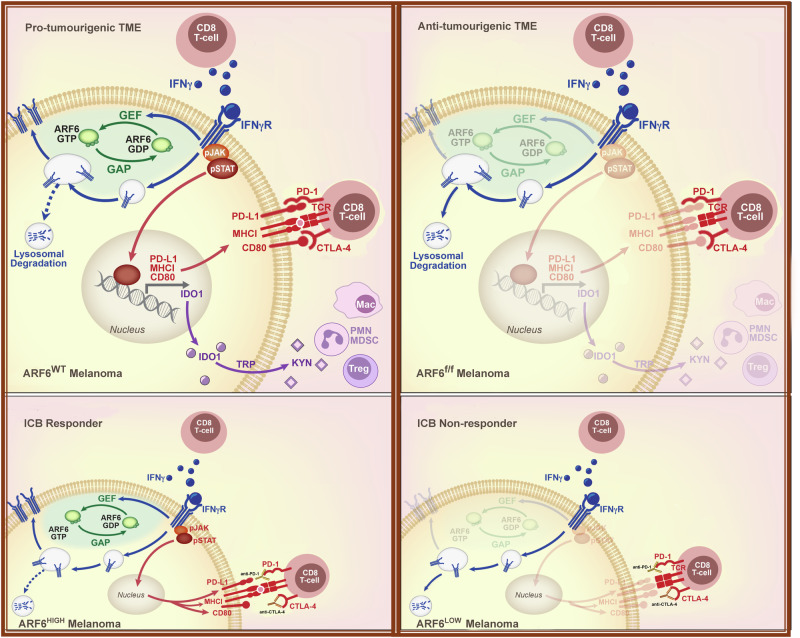
Fig. 7. Proposed model of ARF6-dependent immune suppression by melanoma.
Top panels illustrate an ARF6-dependent mechanism of TME remodelling that accelerates tumour development. ARF6 is activated by IFNγ in a positive feedback loop that diverts the IFNγ receptor away from the lysosome, back to the plasma membrane, augmenting tumourintrinsic, IFNγ-induced expression of checkpoint ligands, PD-L1 and CD80, which inhibit CD8+ T cells that synapse with tumour antigen-loaded MHC-I. ARF6 activation also enhances expression of IFNγ-induced IDO-1, which facilitates recruitment and/or activation of immunosuppressive regulatory T cells (Tregs) and polymorphonuclear-derived myeloid-derived suppressor (PMN-MDSC) cells. Loss of ARF6 diminishes IFNγ-dependent immune suppression, unleashes CD8+ T cell effector function, and restricts tumour development. Bottom panels illustrate the impact of tumour-intrinsic ARF6 on response to immune checkpoint blockade therapy (ICB). Tumours with relatively high ARF6 expression/activation are reliant on IFNγ-driven immune resistance and are vulnerable to ICB. In contrast, tumours with relatively low ARF6 expression/activation progress independent of IFNγ-induced checkpoints and are resistant to therapy.