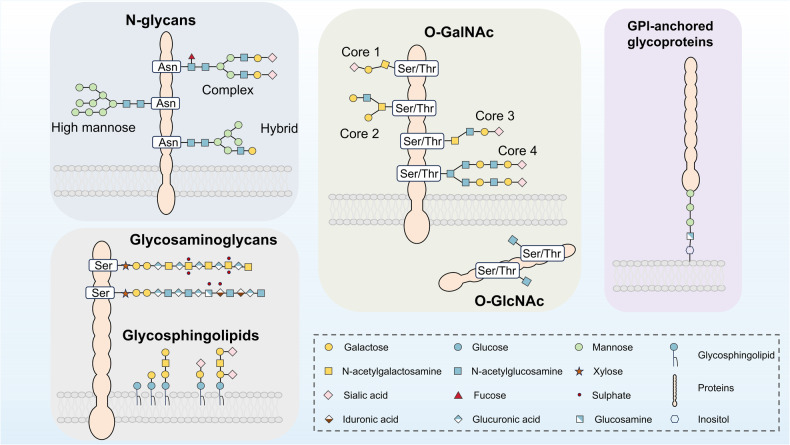
Fig. 2.
Classification of major manifestations of glycosylation on proteins. Glycosylation of proteins is capable of occurring when a saccharide is covalently attached to the polypeptide backbone via N-linkage to Asn or O-linkage to Ser/Thr. N-glycans that have complex, hybrid, or high mannose forms, are linked to Asn via GlcNAc. GalNAc links O-glycans to Ser/Thr with a variety of core structures and extensions, most of which are sialylated and fucosylated. Single GlcNAc molecules are linked to the Ser/Thr residue of intracellular proteins in the cytoplasm, mitochondria, and nucleus through the process of O-GlcNAc glycosylation. Certain glycoproteins known as glycosylphosphatidylinositol (GPI)-anchored proteins are also present in the plasma membrane’s outer leaflet and are connected to a phosphatidylinositol. A significant portion of the cell plasma membrane’s outer leaflet is made up of glycosphingolipids. Terminal sialic acids can be used to further modify the various series of structures that make up these ceramide-linked glycans. GalNAc, N-acetyl-d-galactosamine; GlcNAc, N-acetyl-d-glucosamine; GPI, glycosylphosphatidylinositol