Abstract
Background
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a primary arrhythmia disorder characterized by syncope or sudden cardiac death and typically caused by a gain-of-function of the Ryanodine Receptor Type 2 (RyR2) mutation. Calmodulin is a calcium-binding protein responsible for many intracellular signalling pathways and disruptions in function or regulation may lead to potentially fatal arrhythmias. We present a case of a young patient with CPVT found to have an unusual, potentially causative, Calmodulin 2—a protein coding gene (CALM2) mutation.
Case summary
A 21-year-old female with autism was brought to the ED following cardiac arrest. Bidirectional ventricular tachycardia was captured on electrocardiogram. Propranolol was initiated, and patient had no further episodes of ventricular arrhythmia. A subcutaneous implantable cardioverter defibrillator (ICD) was implanted, and further genetics testing was done. Rapid Whole Genome Sequencing (PGnome®—RAPID) resulted heterozygous variant of uncertain significance in CALM2 gene NM_001743.5 for variant c.136G>A.
Discussion
To the authors’ knowledge, this is the third known record of such mutation in accordance with the International Calmodulin Registry (n = 74). Identification of CALM mutations can help advance the understanding of genetic underpinnings of arrhythmias and underscore necessity of genetic screening and personalized treatment strategies. Subcutaneous ICDs offer a promising therapeutic option while minimizing risks associated with traditional transvenous ICDs.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, Cardiac arrest, Bidirectional ventricular tachycardia, Case report
Learning points.
Classical presentations of catecholaminergic polymorphic ventricular tachycardia (CPVT) include syncope, and sometimes in more extreme cases can manifest as cardiac arrest.
In the right clinical context, especially when there is lack of evidence for ischaemia, structural heart disease, or prolonged QT, syncope or cardiac arrest in younger population should increase index of suspicion for CPVT.
Implantable cardioverter defibrillator (ICD) implantation for patients who have already experienced an aborted cardiac arrest is a class I indication. Deciding between transvenous vs. subcutaneous ICD is an individualized process and should involve shared-decision making, particularly in younger patients.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare and potentially fatal genetic disorder characterized by stress-induced ventricular arrhythmias. It affects primarily children and young adults, with an estimated prevalence of 1 in 10 000 individuals.1,2 Mortality of untreated CPVT is alarmingly high, ∼30–50% by the age of 40, due to the risk of sudden cardiac death (SCD), particularly in those undiagnosed or inadequately managed.1–4 Catecholaminergic polymorphic ventricular tachycardia is driven by abnormalities in calcium handling, often triggered by exercise or emotional stress. Diagnostics typically involve exercise stress testing, Holter monitoring, and genetic testing to identify specific mutations.1,3 The most common genetic mutations associated with CPVT include those encoding for cardiac ryanodine receptor (RyR2) and calsequestrin 2 (CASQ2) both of which are involved in calcium storing and signalling cascades.1–3 Standard treatments focus on beta-blockers and implantable cardioverter defibrillators (ICDs) to prevent arrhythmic events, alongside lifestyle modifications to reduce stress and catecholamine surges.1–4 Calmodulin1-3 proteins, also involved in intracellular calcium signalling, are emerging as target of interest in its contribution to the CPVT phenotype.8 There are fewer reports of managements with subcutaneous ICDs. We present a case of a young patient with CPVT found to have an unusual, potentially causative, CALM2 mutation.
Summary figure
Case presentation
A 21-year-old female with autism on risperidone and intellectual disability presented to the hospital following a cardiac arrest. There was no history of prior syncopal events or cardiac arrest, nor any known cardiovascular medical problems. The patient’s parents denied any history of illicit substance or alcohol use. She was at an auto mechanic shop when she appeared to become distressed from surrounding loud noises. The patient became pale and suffered a syncopal event and found to be in ventricular fibrillation (VF). Return of spontaneous circulation was achieved after two rounds of defibrillation. The patient was borderline normotensive at 93/70 mmHg, with a heart rate of 86 beats per minute, and saturating 100% on a bag valve mask An intravenous amiodarone drip at 1 mg/min was started, and oral amiodarone 400 mg was also given. On exam, the patient had Glasgow Coma Scale Score of 1–1–1, was unresponsive with non-purposeful movement and had agonal respirations. Her cardiovascular exam was unremarkable. The patient’s care was continued in the intensive care unit.
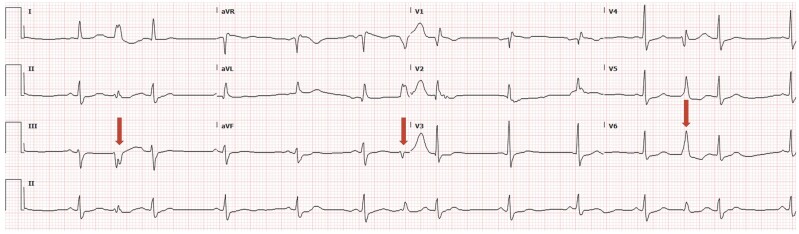
The differential diagnosis included channelopathies, hypertrophic cardiomyopathy, and metabolic abnormalities. Her chemistry panel and electrolytes were normal. Initial electrocardiogram (ECG) (Figure 1) noted sinus rhythm with interpolated premature ventricular complexes and no findings of QT prolongation, infarct, pre-excitation, or Brugada syndrome. The transthoracic echocardiogram (TTE) revealed left ventricular ejection fraction of 45–50% with normal wall thickness and no valvular abnormalities.
Figure 1.
ECG on presentation. Sinus rhythm. Multiple interpolated ventricular premature complexes (arrows). QTC 380 ms. No delta wave or Brugada pattern.
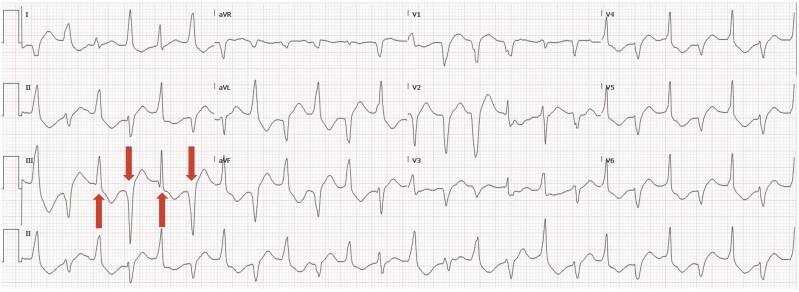
Twenty-four hours later, her ECG evolved into bidirectional ventricular tachycardia (Figure 2). This raised the suspicion for CPVT and thus the Electrophysiology and Genetics teams were consulted. Rapid Whole Genome Sequencing (PGnome®—RAPID) was obtained. Amiodarone was discontinued, and propranolol 10 mg every 6 h was initiated, and no further episodes of ventricular arrhythmia were noted. Vassopressor support was weaned off, and the patient was successfully extubated on hospital Day 4. She returned to her baseline functional status with no significant neurological sequelae. A repeat TTE noted a recovered EF of 55–60% 4 days later.
Figure 2.
ECG on hospital Day 2: bidirectional (beat-to-beat 180° QRS axis) (red arrows) ventricular tachycardia.
The parents expressed concerns that the implantation of hardware would aggravate the patient. The care team explained the necessity of an ICD due to the patient’s near-fatal cardiac arrest and explored options between subcutaneous (SICDs) vs. transvenous (TV-ICDs). After a risks and benefits discussion, the family elected the former as the SICD aligned with their emphasis on a less invasive procedure with potentially fewer complications. A SICD was implanted on hospital Day 10, and the patient was discharged 1 day later with extended-release propranolol 80 mg daily.
Six days post-hospital discharge, the patient and her family followed up with the genetics counselling team. The Rapid Whole Genome Sequencing found a point mutation in the CALM2 gene NM_001743.5 for variant c.136G>A, an amino acid substitution p.Glu46Lys (p.E46K) in the calmodulin protein. The team further explained that although the gene is classified as a variant of uncertain significance, the current literature suggests that disruption in this protein may cause fatal arrhythmias making it the likely genetic culprit in her case.
During her 3-month follow-up, the patient had been adherent to propranolol and was doing well. She has returned to baseline functional status and has had no further episodes of syncope or ICD defibrillation. On routine device interrogation 6 months later, she had no detected episodes of ventricular tachycardia (VT) or VF.
Discussion
Catecholaminergic polymorphic ventricular tachycardia is a channelopathy without structural disease and associated with exercise-induced syncope or SCD, particularly in younger patients. Patients may have normal resting ECGs but exhibit the hallmark finding of bidirectional VT during periods of catecholaminergic surge (e.g. exercise or emotional stress).5,6 Digoxin toxicity or aconitine poisoning may mimic ECG findings. Our case aligns with the current Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society Expert Consensus Statement regarding CPVT diagnosis, as our patient has a structurally normal heart and unexplained bidirectional VT, with an identifiable pathogenic mutation.5,7
Typical forms of CPVT include the dominant disorder arising from cardiac ryanodine receptor (RYR2) mutations and recessive disorder from mutations in the cardiac calsequestrin gene (CASQ2).5,7,8 Atypical forms include mutations involving TRDN or CALM1-3.5,7 Our case focuses on disruptions of the latter, the calmodulin gene. Only recently has CALM2 been observed as potentially pathogenic for CPVT. CALM2 encodes for calmodulin, a protein involved in regulation of calcium-dependent processes. Thus, defects in calmodulin can cause dysfunctional cardiomyocyte calcium homoeostasis, i.e. Ca2+ leakage from sarcoplasmic reticulum.8,9 To our knowledge, this report is the third known record of p.E46K (SupplementalFigure 1) associated with CPVT according to the current International Calmodulin Registry (n = 74), which has classified this variant as ‘Likely Pathogenic.’4 Both cases occurred in males with age of onset between 5 and 10 years and were related to effort-induced polymorphic ventricular tachycardia.8–10
The authors acknowledge the controversy regarding ICD implantation in patients with CPVT, as positive feedback loops may result from ICD shocks and thus have proarrhythmic effects. According to ESC guidelines, for patients who have not had a cardiac arrest, beta-blockers, flecainide, or stellate ganglionectomy is recommended.5 However, our patient experienced an aborted cardiac arrest, and thus an ICD implantation remains a class I indication.5 Regarding subcutaneous vs. transvenous (TICD), the former may be an alternative when pacing for bradycardia, cardiac resynchronization, or ATP is not indicated (class IIA).5 Current literature notes that only 12% of ATPs were effective in restoring sinus rhythm in patients with CPVT.11,12 Typically, spontaneous RR interval variabilities are more likely ATP-responsive, whereas greater variation in QRS morphology is less responsive, likely attributed to the lack of organized re-entry, and therefore rarely interrupted by pacing.11,12 A subcutaneous ICD was chosen in light of the patient’s age, to preserve venous vasculature, and to reduce the risk of long-term complications.13–15 Pooled analysis specifically regarding SICD in patients with CPVT has noted a high complication-free rate (>98%) after one year.13,14 Both TICDs and SICDs have demonstrated issues with inappropriate shocks.13,14
The detection of CALM mutations aids in bolstering the genetic foundations of arrhythmias, stressing the necessity of genetic screening and individualized treatment plans. Our case helps demonstrate that SICDs may be a viable option, especially for younger patients impacted by CPVT.
Supplementary Material
Contributor Information
Kimberly R Ding, Department of Internal Medicine, Harbor-UCLA Medical Center, 1000 W Carson St, Torrance, CA 90509, USA.
Angelo L de la Rosa, Department of Cardiology, Harbor-UCLA Medical Center, Torrance, CA, USA.
Duc Do, Department of Electrophysiology, Ronald-Reagan UCLA Medical Center, Los Angeles, CA, USA.
Sonia Shah, Department of Cardiology, Harbor-UCLA Medical Center, Torrance, CA, USA; Department of Cardiology, The Lundquist Institute, Torrance, CA, USA.
Lead author biography

Kimberly Ding is a current internal medicine resident at Harbor-UCLA Medical Center in Torrance, CA, USA. She graduated from University of California, Riverside, School of Medicine. Her current interests include electrophysiology and adult congenital heart disease.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written informed consent for submission and publication of this case report including image and associated text has been obtained from the patient in line with COPE guidance.
Funding: Authors have no funding to declare.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Napolitano C, Mazzanti A, Bloise R, Priori SG. GeneReviews [online]. 2022.
- 2. Mariani MV, Pierucci N, Fanisio F, Laviola D, Silvetti G, Piro A, et al. . Inherited arrhythmias in the pediatric population: an updated overview. Medicina (B Aires) 2024;60:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez-Riera AR, Barbosa-Barros R, de Rezende Barbosa MP, Daminello-Raimundo R, de Lucca AA Jr, de Abreu LC. Catecholaminergic polymorphic ventricular tachycardia, an update. Anna Noninvasive Electrocardiol 2018;23:e12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2012;5:1044–1052. [DOI] [PubMed] [Google Scholar]
- 5. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 6. Baltogiannis GG, Lysitsas DN, Di Giovanni G, Ciconte G, Sieira J, Conte G, et al. . CPVT: arrhythmogenesis, therapeutic management, and future perspectives. A brief review of the literature. Front Cardiovascular Med 2019;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. . HRS/EHS/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRS, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 8. Crotti L, Spazzolini C, Tester DJ, Ghidoni A, Baruteau AE, Beckmann BM, et al. . Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur Heart Journal 2019;40:2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao J, Makiyama T, Yamamoto Y, Kobayashi T, Aoki H, Maurissen TL, et al. . Novel calmodulin variant p. E46K associated with severe catecholaminergic polymorphic ventricular tachycardia produces robust arrhythmogenicity in human induced pluripotent stem cell–derived cardiomyocytes. Circ Arrhythm Electrophysiol 2023;16:e011387. [DOI] [PubMed] [Google Scholar]
- 10. Walsh R, Adler A, Amin AS, Abiusi E, Care M, Bikker H, et al. . Evaluation of gene validity for CPVT and short QT syndrome in sudden arrhythmic death. European Heart J 2022;43:1500–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roses-Noguer F, Jarman JW, Clague JR, Till J. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2014;11:58–66. [DOI] [PubMed] [Google Scholar]
- 12. De Maria E, Giacopelli D, Borghi A, Modonesi L, Cappelli S. Antitachycardia pacing programming in implantable cardioverter defibrillator: a systematic review. World J Cardiol 2017;9:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckert H, El-Battrawy I, Veith M, Roterberg G, Kowitz J, Lang S, et al. . A. Pooled analysis of complications with transvenous ICD compared to subcutaneous ICD in patients with catecholaminergic polymorphic ventricular arrhythmia. J Pers Med 2022;12:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, et al. . Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944–953. [DOI] [PubMed] [Google Scholar]
- 15. Poole JE, Gold MR. Who should receive the subcutaneous implanted defibrillator? The subcutaneous implantable cardioverter defibrillator (ICD) should be considered in all ICD patients who do not require pacing. Circ Arrhythm Electrophysiol 2013;6:1236–1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.