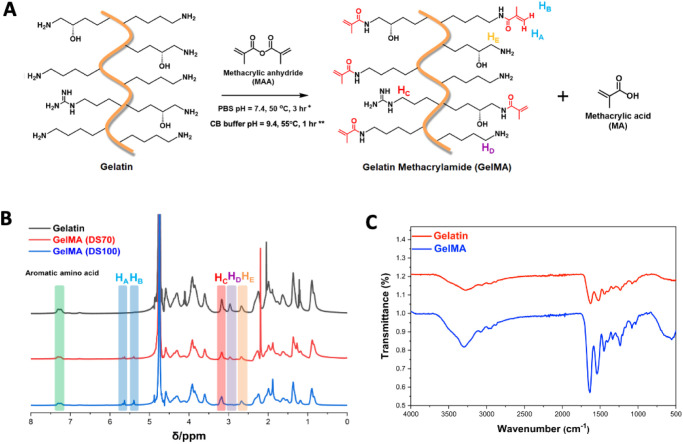
Figure 1.
(A) GelMA synthesized from the reaction of gelatin with methacrylic anhydride (*reaction conditions for DS70 synthesis; **reaction conditions for DS100 synthesis). (B) 1H NMR spectra of gelatin compared to DS70 and DS100 GelMA, confirming the substitution of primary amine groups by methacryloyl groups in GelMA. Specific protons of gelatin and GelMA were highlighted as follows: acrylic protons of methacrylamide groups (HA, HB), methylene protons of arginine residues (HC), methylene protons of lysine residues (HD), and methylene protons of hydroxylysine residues (HE). (C) FTIR spectra of gelatin and GelMA.