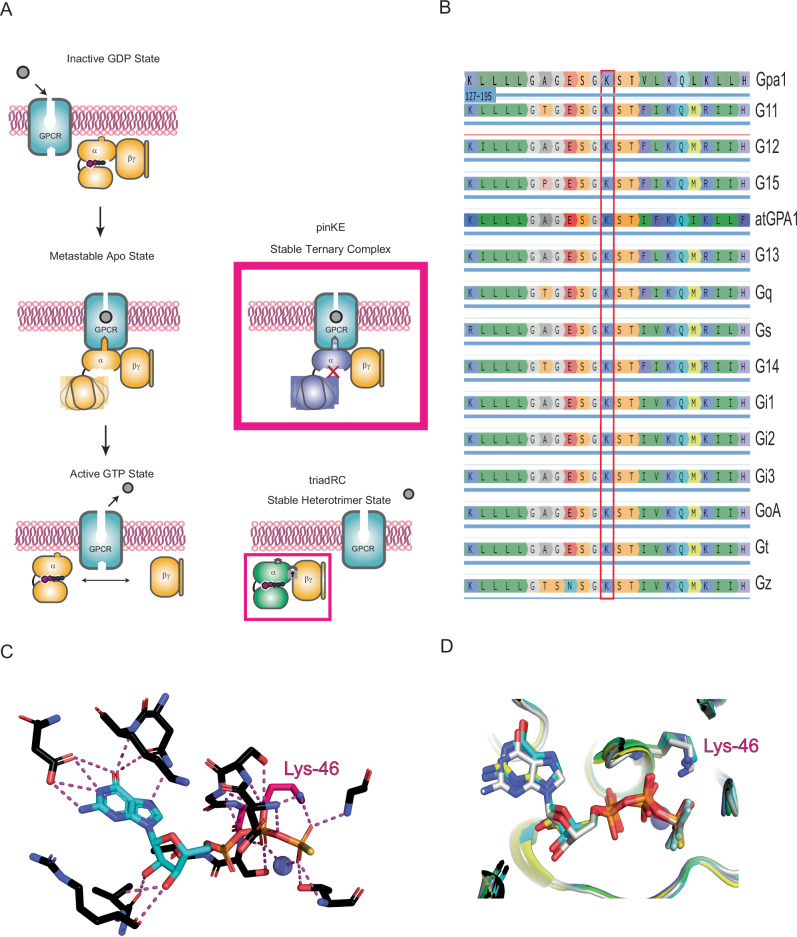
Fig. 1. The phosphate-interacting and neutralizing lysine (pinK) controls one of three binding and unbinding transformations in Gα.
A Agonist binding leads to stabilization of the nucleotide-free form of the G protein heterotrimer, while GTP binding leads to conformational changes in Gα and unbinding of Gα-GTP from the Gβγ subunits. Mutations disrupt GTP binding (pinKE) and GTP-dependent subunit dissociation (triadRC). B The conserved phosphate interacting and neutralizing lysine (pinK) is present in all human Gα proteins as well as Gα proteins from the yeast S. cerevisiae (Gpa1) and plant A. thaliana (AtGPA1). C pinK (Lys-46 in Gαo) forms polar interactions with GTP. D Overlay of multiple Gα structures from PDB ID: 1GIA (Gi1, green), 1AZT (Gs, cyan), 1TND (Gt, blue), 3C7K (Go, gray), and 1ZCA (G12, yellow), showing that pinK bridges the β and γ phosphates of GTP.