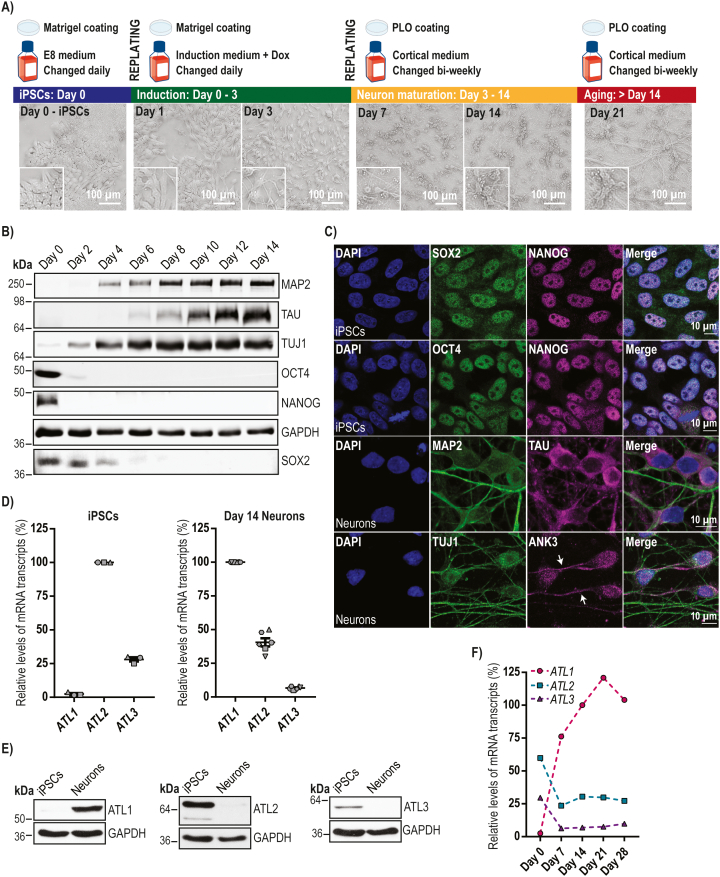
Fig. 1.
Atlastin expression in human iPSC-derived cortical neurons.
A) Schematic diagram of the i3N differentiation protocol, showing the media and well/coverslip coatings that were used during the protocol. The corresponding brightfield microscope images illustrate cellular morphology during the differentiation period. B) i3Ns were incubated with doxycycline for 3 days and cultured for the times indicated. Cells were lysed and immunoblotted with the pluripotency (SOX2, NANOG and OCT4) or neuronal differentiation (MAP2, TAU, TUJ1) markers shown. GAPDH blotting serves as a loading control. C) iPSCs or day 14 i3Ns were fixed, processed for immunofluorescence microscopy and labelled with the pluripotency and neuronal differentiation markers shown. Ankyrin G (ANK3) marks the initial segment of axons (arrows). D) Relative mRNA abundance of atlastin gene transcripts in iPSCs or day 14 i3Ns, measured by qPCR. Atlastin transcript levels were normalised against the ACTB reference gene. Data are displayed relative to the most abundant paralogue in each case. Error bars show mean values +/− SEM of 3 or 6 biological repeats, each carried out in triplicate. E) Immunoblots showing the abundance of atlastin proteins in iPSCs or day 14 i3Ns. GAPDH blotting serves as a loading control. F) The relative mRNA abundance of atlastin transcripts throughout differentiation of i3Ns up to 28 days old, measured by qPCR. ACTB was used to normalise ATL1, −2 and − 3 transcript levels. The data points represent percentages relative to the ATL1 value on day 14. Assays were carried out on one differentiation in triplicate.