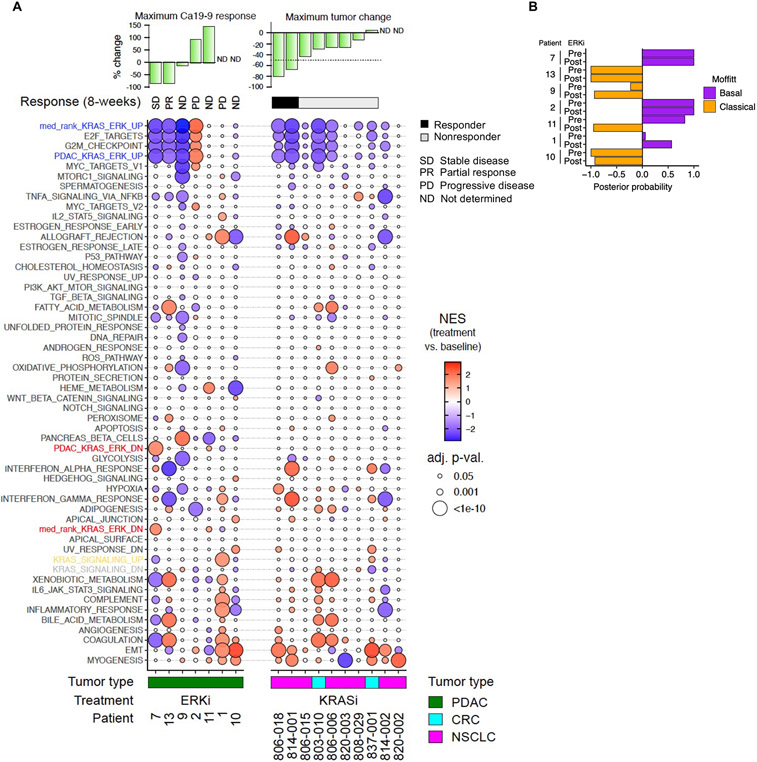
Fig. 5. Changes in KRAS ERK dependent genes coincide with ERKi treatment response in patient tumors.
(A) GSEA evaluation of the median rank KRAS-ERK UP/DN, PDAC KRAS-ERK UP/DN and Hallmark signatures in patient biopsies, pre- and post-treatment. Patients with KRAS-mutant PDAC were treated with ERKi ulixertinib (14 days). Patients with KRASG12C-mutant NSCLC or CRC were treated with the KRASG12C-selective KRASi adagrasib (8 days). The serum biomarker CA 19-9 was used to monitor treatment response, and patient response was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). NES, normalized enrichment score. (B) Evaluation of PDAC subtype in pre- and post-treatment patient biopsies using the Moffitt classifier (23).