Abstract
Objective: To explore the efficacy and safety of tibolone combined with zoledronic acid in the treatment of postmenopausal osteoporosis (PMO). Methods: We conducted a retrospective analysis of 121 PMO patients from March 2019 to July 2021. Patients were divided into two groups based on treatment regimen: an observation group (n=62) receiving zoledronic acid combined with tibolone and a control group (n=59) receiving tibolone monotherapy. We evaluated and compared therapeutic efficacy, bone mineral density, bone metabolism markers (osteocalcin, serum C-terminal telopeptide of type I collagen, and bone alkaline phosphatase), pain, knee joint function, incidence of fragility fractures, and adverse reactions. Logistic regression analysis was used to evaluate risk factors affecting treatment efficacy. Results: The observation group showed a significantly higher therapeutic effect (96.77%) compared to the control group (83.05%), and a lower incidence of fragility fractures (P=0.012). Before treatment, there were no significant differences in bone mineral density, bone metabolism markers, pain status, or knee function between the two groups (all P>0.05). However, after treatment, evaluations showed marked improvements in these parameters in both groups, with more significant enhancements observed in the observation group (all P<0.001). The incidence of adverse reactions did not significantly differ between the groups (20.97% vs 13.56%, P=0.282). Logistic regression analysis identified the use of tibolone combined with zoledronic acid as a protective factor for effective treatment. Conclusions: Tibolone combined with zoledronic acid significantly increases bone mineral density, improves bone metabolism, and reduces pain in PMO patients, with a safety profile comparable to that of monotherapy. This regimen should be considered for clinical use in treating PMO.
Keywords: Tibolone, zoledronic acid, postmenopausal osteoporosis, bone mineral density, bone metabolism
Introduction
As a metabolic skeletal disease, osteoporosis is characterized by decreased bone mass, deterioration of bone tissue microstructure, and increased skeletal fragility, leading to fractures [1]. In China, the prevalence of osteoporosis is rising due to population aging, particularly among postmenopausal women [2]. The decline in ovarian function and rapid estrogen loss result in higher bone resorption than bone formation, thereby increasing osteoporosis incidence [3,4]. Postmenopausal osteoporosis (PMO) significantly affects the quality of life of middle-aged and elderly women [5]. Consequently, delaying osteoporosis progression, reducing fracture risks, and enhancing patient life quality are crucial objectives in healthcare [6].
Currently, clinical treatments for PMO typically involve anti-osteoporosis drugs such as calcium supplements, vitamin D, bisphosphonates, and estrogens [7]. Tibolone, a synthetic 19-carbon steroid, rapidly metabolizes into estrogen and progesterone after ingestion, stabilizing the hypothalamic-pituitary axis and increasing bone mineral density (BMD) [8]. Estrogen promotes osteoblast activity, while progesterone helps maintain peak bone mass [9]. Additionally, tibolone alleviates various symptoms associated with natural and surgical menopause, including hot flashes, mood fluctuations, and night sweats [10]. Zoledronic acid, a third-generation bisphosphonate, not only enhances BMD in postmenopausal women with osteoporosis and fragility fractures but also improves bone metabolism markers and reduces the risk of vertebral fractures [11]. Zoledronic acid is rapidly absorbed by bone tissue, inhibiting bone resorption and increasing BMD [12].
However, the effectiveness of combining tibolone with zoledronic acid for PMO treatment has not yet been explored. Existing clinical approaches mainly focus on monotherapy, with limited research on the necessity and outcomes of combination therapy. This study, therefore, aims to fill this gap by evaluating the efficacy and safety of tibolone and zoledronic acid as combined treatment, thereby providing additional clinical treatment options for PMO.
Materials and methods
Clinical information
Data from 121 patients with PMO treated at Zhejiang Jiashan County First People’s Hospital between March 2019 and July 2021 were retrospectively analyzed. Patients were allocated to either an observation group (receiving combined treatment with zoledronic acid and tibolone; n=62) or a control group (receiving tibolone monotherapy; n=59), based on their treatment regimen.
Inclusion criteria: (1) Diagnosis of osteoporosis and natural menopause for over 12 months; (2) Complete case data; (3) No contraindications to study medications; (4) Absence of other orthopedic diseases; (5) Clear consciousness, and normal cognitive and communication abilities; (6) Complete and standardized medical records, including medical history, laboratory, and imaging results.
Exclusion criteria: (1) Use of other drug therapies within the last six months; (2) Secondary osteoporosis; (3) Severe liver or kidney dysfunction; (4) Autoimmune diseases; (5) Malignant tumors; (6) Incomplete clinical data. The study was approved by the medical ethics committee of Zhejiang Jiashan County First People’s Hospital and adhered to the Declaration of Helsinki.
Treatment methods
All patients received oral calcitriol 0.25 μg/day [Italy HOSPIRA S.P.A. (AbbVie Pte. Ltd.), registration number H20160340] and calcium carbonate D3 tablets 600 mg/day (Wyeth Pharmaceutical Co., Ltd., SFDA Approval No. H10950029). In addition, the control group received oral tibolone 2.5 mg/day (Beijing Zizhu Pharmaceutical Co., Ltd., H20020198) [13]. The observation group was treated with intravenous zoledronic acid 5 mg/year (Switzerland Novartis Pharma Stein AG, registration number H20150049) alongside the calcitriol and calcium carbonate D3 tablets, and oral tibolone was administered at a reduced dose of 1.25 mg/day. Efficacy was evaluated after one year of treatment.
Data collection
Data from eligible patients were collected from medical records and included variables such as age, duration of menopause, body mass index, smoking history, diabetes, hypertension, and year of menopause onset. Post-treatment data collected encompassed treatment efficacy, changes in BMD, bone metabolism markers, the visual analogue scale (VAS) score, the Lysholm knee score, the incidence of fragility fractures, and adverse reactions.
Outcome measures
(1) Therapeutic Effects [14]: Classified into three categories: Markedly Effective: Significant improvement in clinical symptoms, BMD, and bone pain scores post-treatment. Effective: Improvement in clinical symptoms, BMD, and bone pain scores post-treatment. Ineffective: No improvement or worsening of clinical symptoms, BMD, and bone pain scores.
The total response rate was calculated as (number of markedly effective responses + effective responses)/total cases × 100%.
(2) BMD: Measured using dual-energy X-ray absorptiometry (Henan Xinmeikang Trading Co., Ltd., EXA-3000) at the lumbar spine and hip, pre- and post-treatment.
(3) Bone Metabolic Function Indexes: Including osteocalcin, serum C-terminal telopeptide of type I collagen (CTX), and bone alkaline phosphatase (BALP), assessed using enzyme-linked immunosorbent assay kits (Shanghai Qincheng Biotechnology Co., Ltd., QC14314-B, QC14096-A, QC3130-B).
(4) Pain Assessment: Utilizing the VAS [15] pre- and 1 year post-treatment. Scores range from 0-10, with higher scores indicating more severe pain.
(5) Knee Joint Function: Evaluated using the Lysholm knee score [16] which assesses pain, swelling, lameness, and other factors. Scores out of 100, higher scores indicate better function.
(6) Incidence of Fragility Fractures and Adverse Reactions: Both the incidence of fragility fractures and adverse reactions, including headache, gastrointestinal discomfort, muscle and joint pain, and vaginal bleeding, were recorded and compared between the two groups.
Primary outcomes focused on therapeutic effects and changes in BMD. Secondary outcomes assessed changes in osteocalcin, bone metabolic function indexes (CTX and BALP), VAS scores, and the incidence of adverse reactions.
Statistical methods
Data were analyzed using SPSS 19.0, with figures plotted using GraphPad 8. Count data were expressed as percentages and analyzed using the Chi-square test. Continuous data were expressed as Mean ± Standard Error of Mean (SEM), compared between and within groups before and after treatment using independent sample t-tests and paired t-tests, respectively. Logistic regression analysis was used to identify factors influencing treatment efficacy. A p-value <0.05 was considered statistically significant.
Results
Comparison of general information
No significant differences were observed in sex, age, and years of menopause between the groups, ensuring comparability (all P>0.05, Table 1).
Table 1.
Comparison of general information
| Factor | Observation Group (n=62) | Control Group (n=59) | t/χ2 | P |
|---|---|---|---|---|
| Age (year) | 0.030 | 0.864 | ||
| ≤56 | 23 (37.10) | 21 (35.59) | ||
| >56 | 39 (62.90) | 38 (64.41) | ||
| Age (year) | 57.31±8.09 | 57.61±7.62 | 0.210 | 0.834 |
| Duration of menopause (months) | 4.85±2.19 | 4.90±2.45 | 0.118 | 0.906 |
| BMI (Kg/m2) | 0.036 | 0.850 | ||
| ≤23 | 30 (48.39) | 28 (47.46) | ||
| >23 | 32 (51.61) | 31 (52.54) | ||
| BMI (Kg/m2) | 23.27±2.31 | 22.87±1.81 | 1.057 | 0.293 |
| Smoking History | 0.001 | 0.995 | ||
| Yes | 20 (32.26) | 19 (32.20) | ||
| No | 42 (67.74) | 40 (67.80) | ||
| Diabetes | 0.003 | 0.975 | ||
| Yes | 24 (38.71) | 23 (38.98) | ||
| No | 38 (61.29) | 36 (61.02) | ||
| Hypertension | 0.004 | 0.983 | ||
| Yes | 23 (37.10) | 22 (37.29) | ||
| No | 39 (62.90) | 37 (62.71) | ||
| Menopause Years | 0.023 | 0.880 | ||
| ≤4 | 25 (40.32) | 23 (38.98) | ||
| >4 | 37 (59.68) | 36 (61.02) |
Note: BMI, body mass index.
Comparison of therapeutic efficacy
After treatment, the observation group recorded 38 markedly effective, 22 effective, and 2 ineffective cases, whereas the control group had 25 markedly effective, 24 effective, and 10 ineffective cases. The observation group demonstrated significantly higher therapeutic efficacy compared to the control group (P=0.012, 96.77% vs 83.05%, Table 2).
Table 2.
Comparison of therapeutic efficacy
| Therapeutic Efficacy | Observation Group (n=62) | Control Group (n=59) | χ2 | P |
|---|---|---|---|---|
| Markedly Effective | 38 (61.29) | 25 (42.37) | 8.033 | 0.018 |
| Effective | 22 (35.48) | 24 (40.68) | ||
| Ineffective | 2 (3.23) | 10 (16.95) | ||
| Response rate | 60 (96.77) | 49 (83.05) | 6.373 | 0.012 |
Comparison of BMD before and after treatment
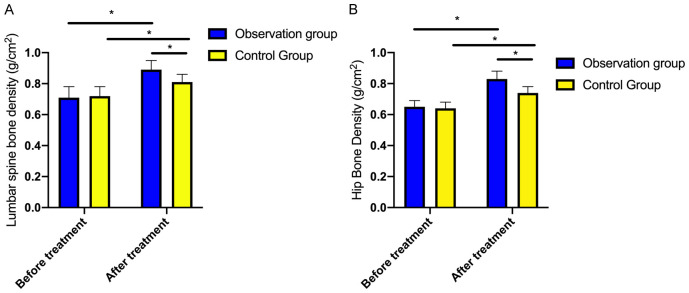
Initially, there was no significant difference in the BMD of the lumbar spine and hip between the two groups (P=0.363). However, both groups exhibited statistically significant increases in BMD after treatment (P<0.001), with more pronounced improvement observed in the observation group (P<0.001, Figure 1).
Figure 1.
Comparison of bone mineral density before and after treatment. A: Comparison of lumbar vertebrae bone mineral density before and after treatment between the two groups of patients; B: Comparison of hip bone mineral density before and after treatment between the two groups of patients. Note: * indicates P<0.05.
Comparison of bone metabolic function indexes before and after treatment
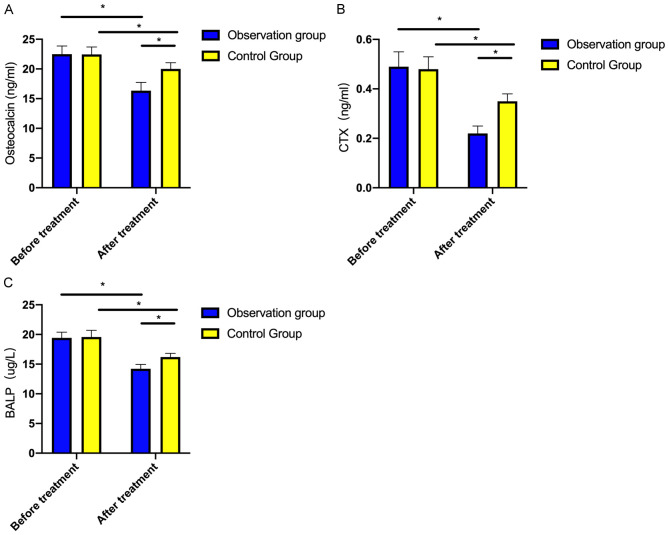
Before treatment, no notable difference was observed in osteocalcin, CTX and BALP between the groups (both P>0.05). Post-treatment, these indexes decreased significantly in both groups, with the observation group showing lower levels compared to the control group (both P<0.001, Figure 2).
Figure 2.
Comparison of bone metabolic function indexes before and after treatment. A: Comparison of osteocalcin before and after treatment; B: Comparison of CTX before and after treatment; C: Comparison of BALP before and after treatment. Note: * indicates P<0.05. CTX, C-terminal telopeptide of type I collagen; BALP, bone alkaline phosphatase.
Comparison of VAS scores before and after treatment
No significant differences were noted in VAS scores between the groups before treatment (P=0.646). However, both groups experienced a decrease in scores after one year of treatment, with a more notable reduction in the observation group (P<0.001, Table 3).
Table 3.
Comparison of VAS scores before and after treatment
| When | Observation Group (n=62) | Control Group (n=59) | t | P |
|---|---|---|---|---|
| Before Treatment | 7.35±0.91 | 7.42±0.75 | 0.460 | 0.646 |
| After Treatment | 1.42±0.50 | 2.46±0.50 | 11.437 | <0.001 |
| t | 44.970 | 42.267 | - | - |
| P | <0.001 | <0.001 | - | - |
Note: VAS, Visual Analogue Scale.
Evaluation of knee joint function before and after treatment
Before treatment, there were no significant differences in Lysholm knee scores between the groups (P=0.464). After one year of treatment, both groups showed increased Lysholm knee scores, with a more substantial improvement observed in the observation group (P<0.001, Table 4).
Table 4.
Comparison of knee joint function before and after treatment
| When | Observation Group (n=62) | Control Group (n=59) | t | P |
|---|---|---|---|---|
| Before Treatment | 46.47±2.51 | 46.14±2.43 | 0.734 | 0.464 |
| After Treatment | 84.95±2.77 | 73.24±3.35 | 20.692 | <0.001 |
| t | 81.056 | 50.298 | - | - |
| P | <0.001 | <0.001 | - | - |
Comparison of incidence of fragility fractures
After treatment, the incidence of fragility fractures was significantly higher in the control group at 20.34% (12/59) compared to 6.45% (4/62) in the observation group (P=0.024, Table 5).
Table 5.
Comparison of incidence of fragility fractures
| Item | Observation Group (n=62) | Control Group (n=59) | χ2 | P |
|---|---|---|---|---|
| Number of Fractures | 4 | 12 | 5.081 | 0.024 |
| Fracture Incidence | 6.45% | 20.34% |
Comparison of incidence of adverse reactions
In the observation group, the incidences of headache, intestinal discomfort, muscle and joint pain, and vaginal bleeding were 3, 3, 4, and 3, respectively. Corresponding data in the control group were 2, 2, 3, and 1. There was no significant increase in the overall incidence of adverse reactions in the observation group compared to the control group (P=0.282, 20.97% vs. 13.56%). All reported symptoms were mild, subsided spontaneously without specific treatment, and did not interfere with ongoing therapy (Table 6).
Table 6.
Comparison of adverse reactions
| Adverse Reactions | Observation Group (n=62) | Control Group (n=59) | χ2 | P |
|---|---|---|---|---|
| Headache | 3 (4.84) | 2 (3.39) | 0.160 | 0.689 |
| Intestinal Discomfort | 3 (4.84) | 2 (3.39) | 0.160 | 0.689 |
| Muscle and Joint Pain | 4 (6.45) | 3 (5.08) | 0.104 | 0.748 |
| Vaginal Bleeding | 3 (4.84) | 1 (1.69) | 0.935 | 0.334 |
| Total Incidence | 13 (20.97) | 8 (13.56) | 1.157 | 0.282 |
Analysis of factors influencing treatment efficacy
Based on treatment outcomes (Table 2), patients were divided into effective (n=109) and ineffective (n=12) groups. A comparative analysis of baseline data revealed significant differences in treatment modalities between these groups (Table 7). Logistic regression analysis identified the combination of tibolone and zoledronic acid as a protective factor for effective treatment (Table 8).
Table 7.
Univariate analysis affecting treatment efficacy
| Effective Group (n=109) | Ineffective Group (n=12) | χ2/t | P | |
|---|---|---|---|---|
| Age (year) | 0.028 | 0.867 | ||
| ≤56 | 39 (35.78) | 4 (33.33) | ||
| >56 | 70 (64.22) | 8 (66.67) | ||
| Age (year) | 57.54±7.66 | 56.83±8.90 | 0.299 | 0.765 |
| Duration of menopause (months) | 4.86±2.27 | 5.00±2.80 | 0.195 | 0.846 |
| BMI (kg/m2) | 1.269 | 0.260 | ||
| ≤23 | 55 (50.46) | 4 (33.33) | ||
| >23 | 54 (49.54) | 8 (66.67) | ||
| BMI (Kg/m2) | 0.072 | 0.943 | ||
| Smoking History | 0.007 | 0.931 | ||
| Yes | 35 (32.11) | 4 (33.33) | ||
| No | 74 (67.89) | 8 (66.67) | ||
| Diabetes | 0.698 | 0.403 | ||
| Yes | 41 (37.61) | 6 (50.00) | ||
| No | 68 (62.39) | 6 (50.00) | ||
| Hypertension | 2.579 | 0.110 | ||
| Yes | 38 (34.86) | 7 (58.33) | ||
| No | 71 (65.14) | 5 (41.67) | ||
| Menopause Years | 1.939 | 0.164 | ||
| ≤4 | 41 (37.61) | 7 (58.33) | ||
| >4 | 68 (62.39) | 5 (41.67) | ||
| Index level before treatment | ||||
| VAS | 7.41±0.86 | 7.17±0.83 | 0.941 | 0.349 |
| Joint function | 46.35±2.46 | 45.58±2.47 | 1.022 | 0.309 |
| Vertebrae bone mineral density (g/cm2) | 0.71±0.08 | 0.68±0.05 | 1.612 | 0.110 |
| Hip bone mineral density (g/cm2) | 0.64±0.06 | 0.66±0.08 | 0.938 | 0.350 |
| Osteocalcin (ng/ml) | 23.48±2.63 | 23.41±1.60 | 0.096 | 0.924 |
| CTX (ng/ml) | 0.50±0.07 | 0.51±0.08 | 0.527 | 0.599 |
| BALP (µg/L) | 19.10±1.37 | 19.39±2.16 | 0.665 | 0.507 |
| Treatment approach | 6.373 | 0.012 | ||
| Tibolone | 49 | 10 | ||
| Tibolone + zoledronic acid | 60 | 2 |
Note: VAS, Visual Analogue Scale; BMI, body mass index; CTX, C-terminal telopeptide of type I collagen; BALP, bone alkaline phosphatase.
Table 8.
Logistic regression analysis affecting treatment efficacy
| β | SE | Wald | P | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Treatment approach (0 = Tibolone, 1 = Tibolone + zoledronic acid) | 1.812 | 0.798 | 5.154 | 0.023 | 6.122 | 1.281-29.264 |
| Constant | 1.589 | 0.347 | 20.976 | 0.000 | 4.900 | - |
Discussion
Osteoporosis, a disease that predominantly affects elderly and postmenopausal women, is characterized by joint pain, limited mobility, and varying degrees of fractures [17], significantly compromising patient quality of life [18]. It is crucial to analyze the underlying causes of PMO and implement effective treatment.
For the treatment of PMO, clinically used anti-osteoporosis drugs include calcium preparations, vitamin D, bisphosphonates, and estrogens [19]. Tibolone, a synthetic 19-carbon steroid, is metabolized into estrogen and progesterone after absorption, although it initially lacks effective hormonal activity. Its therapeutic effects are mediated through the activity of specific enzymes in different tissues, allowing it to bind to corresponding receptors and antagonize bone resorption [20,21]. Zoledronic acid, a bisphosphonate, inhibits osteoclast activity, exerts anti-resorptive effects, and enhances BMD [11]. Previous studies have shown that zoledronic acid prevents bone mineral mass loss following oophorectomy [22].
Currently, PMO is predominantly treated with monotherapy, highlighting a gap in the analysis and evaluation of the efficacy and safety of combination therapies. Our study suggests that the combined therapy of tibolone and zoledronic acid significantly improves bone metabolism and provides superior therapeutic effects compared to tibolone monotherapy. Various treatment options are recommended for postmenopausal women with osteoporosis, including selective estrogen receptor modulators (SERMs), bisphosphonates, parathyroid hormone peptides, denosumab, romosozumab, and other pharmacological interventions like hormone therapy [23]. Hormone therapy is effective in preventing accelerated bone turnover and loss across all skeletal sites and reduces the risk of both vertebral and nonvertebral fractures, regardless of baseline BMD. However, due to estrogen sensitivity leading to breast and endometrial cancers and adverse effects such as weight gain, bloating [24], and unexpected bleeding [25], hormone therapy has specific contraindications. In contrast, tibolone, with a structure distinct from estrogens and SERMs, offers efficacy comparable to conventional hormone therapy without stimulating the breast and endometrium, and it induces less unscheduled bleeding [26]. Research indicates that tibolone is a viable alternative for managing menopausal symptoms and protecting bone health [27].
Additionally, compared to the control group, patients in the observation group exhibited lower VAS scores, higher Lysholm scores, and increased BMD of the lumbar vertebral bodies (L1-4) and the femoral neck and tuberosity. These findings suggest that the combination of tibolone and zoledronic acid can effectively reduce pain, enhance knee joint function, and increase BMD. The mechanism behind these effects likely involves zoledronic acid’s inhibition of the mevalonate pathway within cells, which inactivates guanosine triphosphatases that regulate osteoclast morphology and cytoskeletal arrangement. This inhibition is due to its effect on a critical regulatory enzyme, farnesyl pyrophosphate synthase, leading to reduced osteoclast activity and promoted apoptosis. Furthermore, zoledronic acid prevents the resorption of cartilage and immature bone tissue by osteoclasts, thereby alleviating pain and treating osteoporosis [28]. Other studies corroborate these benefits, with reports of significant improvements in bone mass, ambulation, low back pain, and daily life activities in patients treated with zoledronic acid [29]. Additionally, it has been found that tibolone does not interfere with the effects of zoledronic acid [30]. Notably, zoledronic acid’s inhibitory effect on bone resorption is stronger than that of alendronate and requires only annual intravenous administration. Once administered, the drug rapidly distributes to bone tissues, demonstrating high affinity for bone mineral and pyrophosphate synthase. Given its rapid onset, potent efficacy, and prolonged action, zoledronic acid represents a unique bisphosphonate that is administered just once a year intravenously [31]. Our study is the first to demonstrate the beneficial clinical efficacy of combined tibolone and zoledronic acid treatment for PMO.
In terms of preventing fragility fractures, a significantly lower incidence was observed in patients receiving combined treatment. This benefit likely results from the dual-drug approach which reduces bone turnover markers, enhances bone metabolic function, increases BMD, and decreases bone loss, thereby lowering fracture risk. Although the incidence of adverse reactions in the combination therapy group was not significantly different from that in the monotherapy group, patients receiving combination therapy tended to experience more adverse reactions. This suggests that the use of combination or sequential therapy should be carefully considered during treatment. However, most adverse reactions were mild and resolved following drug withdrawal and symptomatic treatment. Given that the benefits of combined treatment outweigh the risks, it is advisable to encourage patients to adhere to the treatment and manage adverse reactions proactively to achieve optimal therapeutic outcomes.
Nevertheless, this study has certain limitations. Firstly, due to the limited sample size, patients were only divided into two groups (tibolone monotherapy vs. combination therapy), which leaves room for further validation of our conclusions. Secondly, the findings from this study cannot be generalized to women with osteoporosis or previous fractures, as this population may respond differently to antiresorptive agents with a greater increase in BMD. Future studies will expand the sample size and include a broader range of therapeutic drugs to provide a more comprehensive comparison of the efficacy of combined therapy in PMO patients and further discuss its clinical implications.
In conclusion, the combination of tibolone and zoledronic acid is effective in treating PMO, significantly improving patients’ bone metabolism and BMD, and reducing the incidence of fractures. With a manageable safety profile, this treatment regimen is worthy of clinical consideration and broader adoption.
Disclosure of conflict of interest
None.
References
- 1.Miller PD. Management of severe osteoporosis. Expert Opin Pharmacother. 2016;17:473–488. doi: 10.1517/14656566.2016.1124856. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC, Lin TC, Lange JL, Chen L, Wong ICK, Sing CW, Cheung CL, Shao SC, Yang YK. Effectiveness of denosumab for fracture prevention in real-world postmenopausal women with osteoporosis: a retrospective cohort study. Osteoporos Int. 2022;33:1155–1164. doi: 10.1007/s00198-021-06291-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM. The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging. 2015;10:583–591. doi: 10.2147/CIA.S54614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5:908–923. doi: 10.1016/S2213-8587(17)30184-5. [DOI] [PubMed] [Google Scholar]
- 5.Anupama DS, Norohna JA, Acharya KK, Ravishankar, George A. Effect of exercise on bone mineral density and quality of life among postmenopausal women with osteoporosis without fracture: a systematic review. Int J Orthop Trauma Nurs. 2020;39:100796. doi: 10.1016/j.ijotn.2020.100796. [DOI] [PubMed] [Google Scholar]
- 6.North American Menopause Society. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause. 2006;13:340–369. doi: 10.1097/01.gme.0000222475.93345.b3. quiz 368-9. [DOI] [PubMed] [Google Scholar]
- 7.Brown JP. Long-term treatment of postmenopausal osteoporosis. Endocrinol Metab (Seoul) 2021;36:544–552. doi: 10.3803/EnM.2021.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ettinger B. Tibolone for prevention and treatment of postmenopausal osteoporosis. Maturitas. 2007;57:35–38. doi: 10.1016/j.maturitas.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 10.Duralde ER, Sobel TH, Manson JE. Management of perimenopausal and menopausal symptoms. BMJ. 2023;382:e072612. doi: 10.1136/bmj-2022-072612. [DOI] [PubMed] [Google Scholar]
- 11.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 12.Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A, Wang C, Wagman RB, Cummings SR. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101:3163–3170. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao RX, Yu M, Jiang Y, Xia W. Management of osteoporosis with calcitriol in elderly Chinese patients: a systematic review. Clin Interv Aging. 2014;9:515–526. doi: 10.2147/CIA.S40465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong L, Zuo K, Ma L. Clinical effect of zoledronic acid in the treatment of senile osteoporosis. Pak J Med Sci. 2020;36:1703–1707. doi: 10.12669/pjms.36.7.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi N, Zhao C, Fang C, Zhang D, Zhou Z, Ouyang G. Effects of acupoint catgut embedding on the postmenopausal osteoporosis patients and related mechanism. Am J Transl Res. 2021;13:1789–1798. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kang SB, Nam K, Rhee SH, Won JW, Han HS. Retrograde intramedullary nailing for distal femur fracture with osteoporosis. Clin Orthop Surg. 2012;4:307–312. doi: 10.4055/cios.2012.4.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhammad A, Mada SB, Malami I, Forcados GE, Erukainure OL, Sani H, Abubakar IB. Postmenopausal osteoporosis and breast cancer: the biochemical links and beneficial effects of functional foods. Biomed Pharmacother. 2018;107:571–582. doi: 10.1016/j.biopha.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 19.Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem. 2018;119:4469–4481. doi: 10.1002/jcb.26550. [DOI] [PubMed] [Google Scholar]
- 20.Formoso G, Perrone E, Maltoni S, Balduzzi S, Wilkinson J, Basevi V, Marata AM, Magrini N, D’Amico R, Bassi C, Maestri E. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev. 2016;10:CD008536. doi: 10.1002/14651858.CD008536.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Río JP, Molina S, Hidalgo-Lanussa O, Garcia-Segura LM, Barreto GE. Tibolone as hormonal therapy and neuroprotective agent. Trends Endocrinol Metab. 2020;31:742–759. doi: 10.1016/j.tem.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Yunus F, Bell R, Body J, Quebe-Fehling E, Seaman J. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J. Clin. Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 23.Rizzoli R. Postmenopausal osteoporosis: assessment and management. Best Pract Res Clin Endocrinol Metab. 2018;32:739–757. doi: 10.1016/j.beem.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 24.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728–753. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 25.de Medeiros SF, Yamamoto MM, Barbosa JS. Abnormal bleeding during menopause hormone therapy: insights for clinical management. Clin Med Insights Womens Health. 2013;6:13–24. doi: 10.4137/CMWH.S10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieciecka A, Kędziora-Kornatowska K, Janiszewska M. Tibolone among drugs in the therapy of postmenopausal women. Med Res J. 2021;6:140–146. [Google Scholar]
- 27.Castrejón-Delgado L, Castelán-Martínez OD, Clark P, Garduño-Espinosa J, Mendoza-Núñez VM, Sánchez-Rodríguez MA. Effect of tibolone on bone mineral density in postmenopausal women: systematic review and meta-analysis. Biology (Basel) 2021;10:211. doi: 10.3390/biology10030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostyshyn NM, Gzhegotskyi MR, Kostyshyn LP, Mudry SI. Effect of zoledronic acid on bone nanocomposites organization and prevention of bone mineral density loss in ovariectomized rats. Drug Metab Pers Ther. 2021;36:239–245. doi: 10.1515/dmpt-2020-0187. [DOI] [PubMed] [Google Scholar]
- 29.Cauley JA, Black D, Boonen S, Cummings SR, Mesenbrink P, Palermo L, Man Z, Hadji P, Reid IR HORIZON Pivotal Fracture Group. Once-yearly zoledronic acid and days of disability, bed rest, and back pain: randomized, controlled HORIZON Pivotal Fracture Trial. J Bone Miner Res. 2011;26:984–992. doi: 10.1002/jbmr.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien YTK, Madrasi K, Samant S, Kim MJ, Li F, Li L, Wang Y, Schmidt S. Establishment of a disease-drug trial model for postmenopausal osteoporosis: a zoledronic acid case study. J Clin Pharmacol. 2020;60(Suppl 2):S86–S102. doi: 10.1002/jcph.1748. [DOI] [PubMed] [Google Scholar]
- 31.Recker RR, Delmas PD, Halse J, Reid IR, Boonen S, García-Hernandez PA, Supronik J, Lewiecki EM, Ochoa L, Miller P, Hu H, Mesenbrink P, Hartl F, Gasser J, Eriksen EF. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res. 2008;23:6–16. doi: 10.1359/jbmr.070906. [DOI] [PubMed] [Google Scholar]