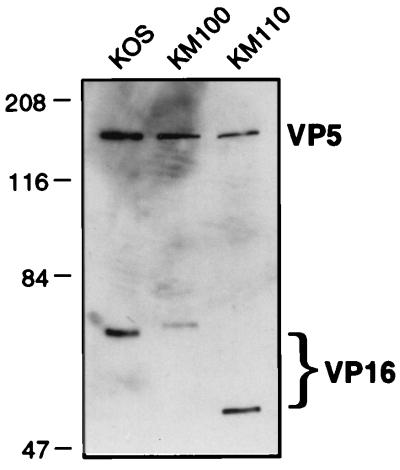
FIG. 5.
VP16 levels in purified virions. KOS, KM100, and KM110 virions were purified by centrifugation through dextran gradients (see Materials and Methods), pelleted, resuspended in SDS-PAGE sample buffer, and subjected to electrophoresis through an SDS–9% polyacrylamide gel. The relative amounts of the capsid protein (VP5) and VP16 were then visualized by Western blot analysis with antibodies NC-1 and LP1, respectively. Note the differences in the electrophoretic mobilities of the wild-type, in1814, and V422 forms of VP16.