Abstract
Late sleep timing is prevalent in early childhood and a risk factor for poor behavioral and health outcomes. Sleep timing is influenced by the phase of the circadian clock, with later circadian timing linked to delayed sleep onset in young children. Light is the strongest zeitgeber of circadian timing and, in adults, evening light produces circadian phase delay in an intensity-dependent manner. The intensity-dependent circadian phase-shifting response to evening light in children, however, is currently unknown. In the present study, 33 healthy, good sleeping children aged 3.0-4.9 years (M = 4.14 years, 39% male) completed a 10-day between-subjects protocol. Following seven days of a stable sleep schedule, an in-home dim-light circadian assessment was performed. Children remained in dim-light across 3 days (55h), with salivary melatonin collected in regular intervals throughout each evening. Phase shifting effects of light exposure were determined via changes in the timing of the dim-light melatonin onset (DLMO) prior to (Day 8) and following (Day 10) a light exposure stimulus. On Day 9, children were exposed to a 1-h light stimulus in the hour before their habitual bedtime. Each child was randomly assigned to one intensity between 5-5,000 lux (4.5-3,276 melanopic EDI). Across light intensities, children showed significant circadian phase delays, with an average phase delay of 56.1 min (SD = 33.6 min), and large inter-individual variability. No relationship between light intensity and magnitude of the phase shift was observed. However, a greater percentage of melatonin suppression during the light exposure was associated with a greater phase delay (r = −0.73, p < 0.01). These findings demonstrate that some young children may be highly sensitive to light exposure in the hour before bedtime and suggest that the home lighting environment and its impact on circadian timing should be considered as a possible contributor to behavioral sleep difficulties.
Keywords: Circadian rhythm, light, phase delay, sleep, child development, preschool
Introduction
Behavioral sleep problems (e.g., bedtime resistance, difficulty falling asleep, late sleep onset) are widespread in early childhood, impacting approximately 30% of young children (Owens, 2008; Simola et al., 2010; Tikotzky & Sadeh, 2001). Childhood sleep problems peak in prevalence during the preschool years and are an emerging risk factor for poor behavioral and chronic health outcomes (Beltramini & Hertzig, 1983; Gregory et al., 2005; Gregory, Eley, O’Connor, & Plomin, 2004; Gregory & O’Connor, 2002; Mindell, Kuhn, Lewin, Meltzer, & Sadeh, 2006). Such problems often persist throughout the school-aged years and are associated with concurrent and future attentional, emotional, and cognitive difficulties, as well as an increased risk of childhood obesity (Bruni, Reto, Miano, & Ottaviano, 2000; Jiang et al., 2008; Lavigne et al., 1999; Li, Zhang, Huang, & Chen, 2017; Maski & Kothare, 2013).
The timing of sleep is influenced by the phase of the circadian clock. Later circadian timing in young children is linked to increased behavioral sleep problems and delayed sleep onset (LeBourgeois, Carskadon, et al., 2013; LeBourgeois, Wright Jr, LeBourgeois, & Jenni, 2013; Simpkin et al., 2014). Although adults typically go to bed ~2 h after their melatonin onset (Burgess & Eastman, 2005; Wright, Gronfier, Duffy, & Czeisler, 2005), young children generally do not choose their own bedtimes. Parent selected bedtimes occur an average of 48 min after melatonin onset in preschoolers (LeBourgeois, Carskadon, et al., 2013). A decrease in the window between children’s melatonin onset and scheduled bedtime is associated with longer sleep-onset latencies and increased bedtime resistance, demonstrating that dissonance between parent-selected bedtimes and children’s circadian timing can contribute to later sleep timing in early childhood (LeBourgeois, Wright Jr, et al., 2013).
Light is the strongest zeitgeber of circadian timing in humans. Even low intensities of light can suppress evening melatonin levels, as well as delay circadian timing in adults (Duffy & Wright, 2005; Zeitzer, Dijk, Kronauer, Brown, & Czeisler, 2000). Light may be a particularly relevant factor for determining sleep and circadian timing in early childhood. In preschoolers, greater ambient light levels in the 2 h before bedtime are associated with later circadian timing when controlling for bedtime (Akacem, Wright Jr, & LeBourgeois, 2016). Also, the melatonin suppression response in young children is highly sensitive to evening light exposure. We previously demonstrated that preschool-aged children had robust and sustained suppression of melatonin in response to a bright (1000 lux) 1-h light exposure in the hour before bedtime (Akacem, Wright, & LeBourgeois, 2018). Further, we previously reported high suppression of melatonin across a wide range of intensities and regardless of the individual circadian timing of the light exposure (Hartstein et al., 2022). Additionally, previous findings indicate that children’s circadian systems are more sensitive to evening light than those of adults (Higuchi, Nagafuchi, Lee, & Harada, 2014). This may be due in part to developmental changes in eye anatomy. School-aged children have larger pupils than adults under both dim and bright light conditions, as well as higher lens transmittance, which translates to significantly greater non-visual photoreception and light-induced melatonin suppression (Eto et al., 2021; Higuchi et al., 2014). Together, these findings indicate that children possess a heightened light-induced melatonin suppression response throughout much of development.
In young and older adults, light at night delays circadian phase in an intensity-dependent manner (Duffy & Wright, 2005; Gooley et al., 2010; Zeitzer et al., 2000). Employing a 6.5-h nighttime light exposure stimulus, Zeitzer and colleagues established an illuminance-response curve in young adults over a range of intensities from 3 to 9100 lux. In this study, 50% of the maximal phase delay was observed after an exposure to approximately 100 lux, which is within the range of typical indoor room lighting (Zeitzer et al., 2000). Duffy and colleagues (2005) also showed that light at night delays circadian phase in older adults in an intensity-dependent manner over a range of intensities from 2 to 8000 lux. The sensitivity to light was reduced in older compared to younger adults. The sensitivity of the circadian phase shifting response to evening light exposure in early childhood has yet to be examined. Such data are critical to defining developmentally sensitive lower and saturating limits of light to produce phase shifts in young children and to make meaningful clinical recommendations to parents and health care professionals about the intensity of evening light in order to support children’s sleep and circadian rhythms.
The present study utilized a 10-day experimental protocol with a 7-day stable sleep schedule followed by a 3-day circadian protocol to establish the impact of evening light intensity on circadian phase shifts in preschool-aged children. Children completed circadian phase assessments on the first and last nights of the in-home protocol to quantify the circadian phase shift response of the dim-light melatonin onset (DLMO). On the night between these assessments, children were exposed to a 1-h light exposure with a randomly assigned light intensity between 5-5,000 lux in the hour before habitual bedtime. We hypothesized that, like in adults, evening light exposure would delay circadian timing in a non-linear, intensity-dependent manner.
Methods
Participants
33 healthy, good-sleeping children (39% male, 97% Caucasian, 3% Mixed Race) aged 3.0-4.9 years (M = 4.1, SD = 0.5 years) completed the study protocol. An additional 5 children were enrolled in the study but did not complete the protocol due to child illness (n=1) or accidental light exposure during the in-home protocol (n=4). Participants were recruited from the greater Boulder/Denver, CO area through flyers, community events, local preschools, and a previously established database of interested families. Eligibility was confirmed through a series of online questionnaires and an in-depth phone interview with parents. Exclusion criteria included parent report of any of the following: clinical sleep disorders; behavioral problems; developmental disorders; metabolic disorders; chronic medical conditions; head injury involving loss of consciousness; migraines or frequent headaches; oral disease or injury; pre- or post-term delivery (term = 35-45 weeks) or low birth weight (<5.5 lbs); current use of medication affecting sleep, circadian rhythms, or light sensitivity; visual impairment or color blindness; travel beyond 2 time zones in the 2 months prior to the assessment; parent reported sleep schedule varying >2 h between weekdays and weekends; or regular daytime napping (>2 times per week). All study procedures were approved by the University of Colorado Boulder Institutional Review Board. Parents provided written informed consent, and families were compensated for their participation.
Protocol
Children completed a 10-day protocol (Fig 1) during the summer months (May-August) of 2017, 2018, and 2019. For the duration of the protocol, participants wore an actigraph on their non-dominant wrist (Spectrum Plus, Philips Respironics, Pittsburgh, PA, USA) in order to determine sleep/wake timing and measure light exposure. During the first 7 days of the study, children adhered to a strict parent-selected sleep schedule (bedtime and wake time) of at least 10 h time in bed based on the child’s habitual sleep times. Adherence to the protocol and sleep schedules was confirmed through actigraphy, a parent-completed sleep diary, and daily check-ins. The final 3 days consisted of a circadian protocol which took place in participants’ homes (55 h). On Day 8, researchers transformed the home into a dim-light environment, achieving an average light level throughout the home of ~1.5 lux (< 10 lux maximum at the child’s angle of gaze). The participant entered the dim-light environment 4.5 h before their habitual bedtime and remained in dim-light during scheduled wakefulness and darkness during scheduled sleep through the completion of the protocol (2.5 h after habitual bedtime on Day 10). On the evening of Day 8, baseline DLMO was established by collecting saliva samples in 20- to 30-min intervals beginning 3 h and 20 min before habitual bedtime until 1 h past habitual bedtime. On Day 9, participants received a 1-h light exposure in the hour before their habitual bedtime. Children were randomly assigned to a single light intensity between 5 and 5,000 lux in the angle of gaze (See Hartstein, et al. (2022) for the rationale behind the chosen intensities and the photometric properties of each experimental light condition). Children sat at a small table with a flat, tunable LED panel (5000K; Beghelli, USA), and researchers directed children’s gaze downwards to the light source by playing with translucent toys or coloring on transparent overhead sheets. Illuminance at the participant’s angle of gaze was verified with a research photometer (ILT 2400; International Light Technologies, Inc., Peabody, MA, USA) every 10 min during the light exposure, or if the child shifted position, and adjusted accordingly. Saliva samples were collected in 20- to 30-min intervals before, during and after the light exposure beginning 1 h and 20 min before habitual bedtime until 1 h past habitual bedtime. Finally, on Day 10, DLMO was reassessed, collecting saliva samples beginning 3 h and 20 min before habitual bedtime until 2.5 h after habitual bedtime. Samples on Days 9 and 10 were time-locked to those collected on Day 8. If actigraphy from the first 7 days of the protocol revealed a sleep onset latency of >30 min, saliva samples were extended for an additional 60 min (n=2) each evening.
Fig 1: 10-day study protocol.
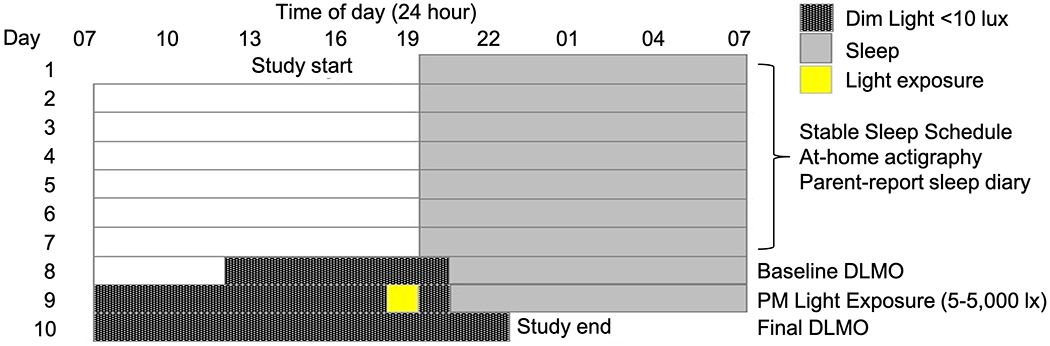
Children followed a strict, parent-selected sleep schedule for 7 days, followed by a 3-day circadian protocol. On Days 8 and 10, DLMO was assessed in order to quantify circadian phase. On Day 9, children were exposed to light for 1 h in the hour before scheduled bedtime. The sleep schedule depicted is an example; actual bedtimes and wake times differed across individual participants.
Saliva was collected by having the participant chew on a braided dental cotton roll for ~2 min. Children remained in a sitting posture for 5 min before, as well as during saliva samples (Deacon & Arendt, 1994) and did not eat or drink for >15 min before each sample. Ambient light levels were measured during each saliva sample by holding the photometer next to the child’s face (~5 cm), pointed in the angle of gaze. Samples were immediately centrifuged and stored in a cooler with ice packs on-site before being returned to the lab each evening and transferred to a −20°C freezer. Samples were later assayed offsite (SolidPhase, Inc., Portland, Maine, USA) by technicians with no knowledge of the study question or conditions.
Analysis
Data from actigraphy were scored using our published standard protocols (LeBourgeois, Carskadon, et al., 2013). Data were aggregated across the first 7 days of actigraphy to compute bedtime (lights-out time), sleep start time, midsleep time (midpoint between sleep start and sleep end), sleep end time, wake time (lights on), and sleep onset latency (number of minutes from bedtime to sleep start). Phase angles were calculated as the difference between the clock time of baseline DLMO and the clock time of each sleep variable (i.e., bedtime, sleep start, midsleep, sleep end, and wake time), as well as the time of the start of the experimental light exposure. Light was also recorded by the actigraph (1-min epochs) and used to calculate average light exposure from wake time to bedtime during each day of the protocol.
Salivary melatonin levels were assayed via radioimmunoassay (Bühlmann Laboratories AG, Schöenbuch, Switzerland). The limits of detection for the assays were 0.5 to 50.0 pg/ml. Samples measured to be >50.0 were recorded as 50.0 pg/ml (n = 22). For samples assayed in 2017 and 2018, inter-assay coefficients of variation (CV) ranged from 11.4% to 12.7%, and intra-assay CV were between 6.3% and 11.0% (n=22). The samples assayed in 2019 had inter-assay CV between 8.2% and 8.7%, and intra-assay CV between 5.0% to 9.9% (n=22). DLMO was calculated as the linear interpolated clock time at which melatonin levels crossed 4 pg/ml, provided they remained above the threshold for at least two consecutive samples (Carskadon, Acebo, Richardson, Tate, & Seifer, 1997; Deacon & Arendt, 1994). One child had consistently high melatonin levels and therefore a threshold of 10 pg/ml was used to calculate DLMO values (Crowley & Eastman, 2017; Hartstein et al., 2022). Analyses were run including and excluding this participant, with no change to the results. See (Hartstein et al., 2022) for calculations and findings on acute melatonin suppression during the light exposure. The circadian phase shift was calculated as the difference between baseline DLMO (Day 8) and final DLMO (Day 10) the evening after the light exposure.
Average melatonin levels on the baseline night (Day 8) and the final night (Day 10) were compared with paired samples t-tests. Differences in phase shift by sex were examined with an independent samples t-test. Bivariate correlations were utilized to examine the association between the magnitude of acute melatonin suppression during the light exposure and the circadian phase shift the following evening, as well as the association between the phase angle of the experimental light exposure and phase shift. All significance testing was conducted with an α -level of 0.05.
Results
A summary of participants’ sleep and circadian variables is presented in Table 1. Baseline DLMO ranged between 17:48 and 21:16, whereas final DLMO ranged from 18:21 to 22:17. Baseline DLMO occurred between 68 min before to 68 min after the start of the experimental light exposure (M = 25.2 min, SD = 34.2 min after light start; Fig 2).
Table 1:
Means and standard deviations of sleep and circadian variables.
| M | SD | |
|---|---|---|
| Sleep variables | ||
| Bedtime | 19:59 | 0:38 |
| Sleep start time | 20:16 | 0:41 |
| Midsleep time | 1:35 | 0:42 |
| Sleep end time | 6:54 | 0:47 |
| Wake time | 7:07 | 0:44 |
| Sleep onset latency (min) | 18.0 | 14.5 |
| Circadian variables | ||
| Baseline DLMO time | 19:27 | 0:49 |
| Final DLMO time | 20:23 | 1:00 |
| Phase Shift (min) | −56.1 | 33.6 |
| Bedtime phase angle (min) | 33.7 | 33.7 |
| Sleep start phase angle (min) | 51.3 | 31.3 |
| Midsleep phase angle (h) | 6.1 | 0.5 |
| Sleep end phase angle (h) | 11.4 | 0.6 |
| Wake time phase angle (h) | 11.6 | 0.6 |
| Light exposure phase angle (min) | 25.1 | 34.1 |
Note: For Baseline DLMO time, Final DLMO time, Phase Shift, and Light exposure phase angle, n = 33. For all other variables, n= 32 due to a technical issue with actigraphy for one participant. Phase angles were calculated in relation to Baseline DLMO.
Fig 2: Distribution of the circadian timing of light onset across participants.
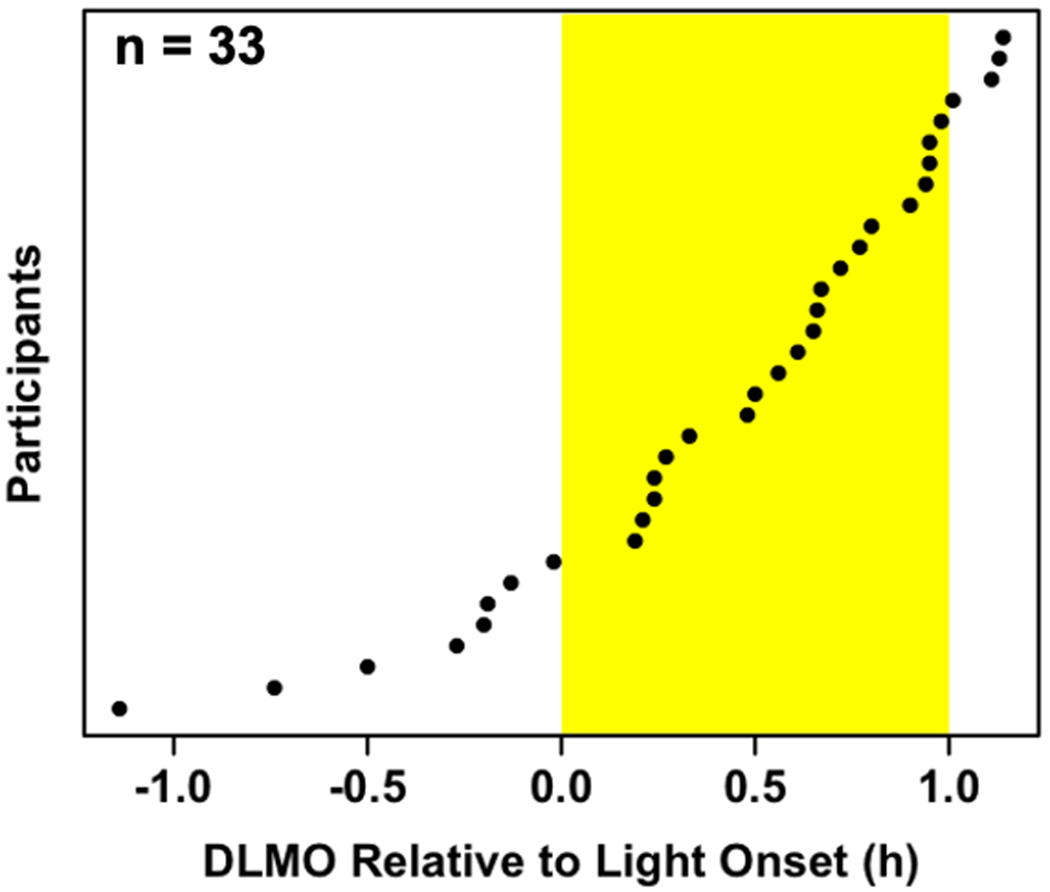
Light onset ranged from 68 min before to 68 min after children’s baseline dim-light melatonin onset.
During the first 7 days of the study protocol, children were exposed to an average of 2,150 lux (SD = 1,573 lux) between wake time and bedtime. During the collection of saliva samples, excluding those collected during the experimental light exposure, the average intensity of light measured next to participants’ eyes was 0.65 lux (SD = 0.20 lux).
Figure 3 depicts group averages of melatonin levels on Days 8 and 10 of the study protocol. Compared to the baseline night (Day 8), average melatonin levels on the final night (Day 10) were lower 50 min (p = 0.01, d = 0.45), 30 min (p < 0.001, d = 0.73), and 10 min (p < 0.001, d = 0.94) before habitual bedtime, as well as 20 min (p < 0.001, d = 0.96) and 50 min (p < 0.001, d = 0.91) after habitual bedtime. Circadian phase shift between Days 8 and 10 ranged from an 8-min phase advance to a 123-min phase delay (Fig 4). The average phase shift across intensities was a 56.1-min delay (SD = 33.6 min). No relationship between the assigned intensity of the light exposure and the magnitude of the resulting phase shift was observed. Phase shift did not differ between male and female participants (t(31) = −0.05, p = 0.96). The phase angle of the light exposure was not correlated with the phase shift response (r = −0.15, p = 0.40). Finally, as depicted in Fig 5, comparable intensities of light at the same circadian time resulted in vastly different magnitudes of phase shift, suggesting individual differences in sensitivity or circadian period.
Fig 3: Group averages of salivary melatonin levels.
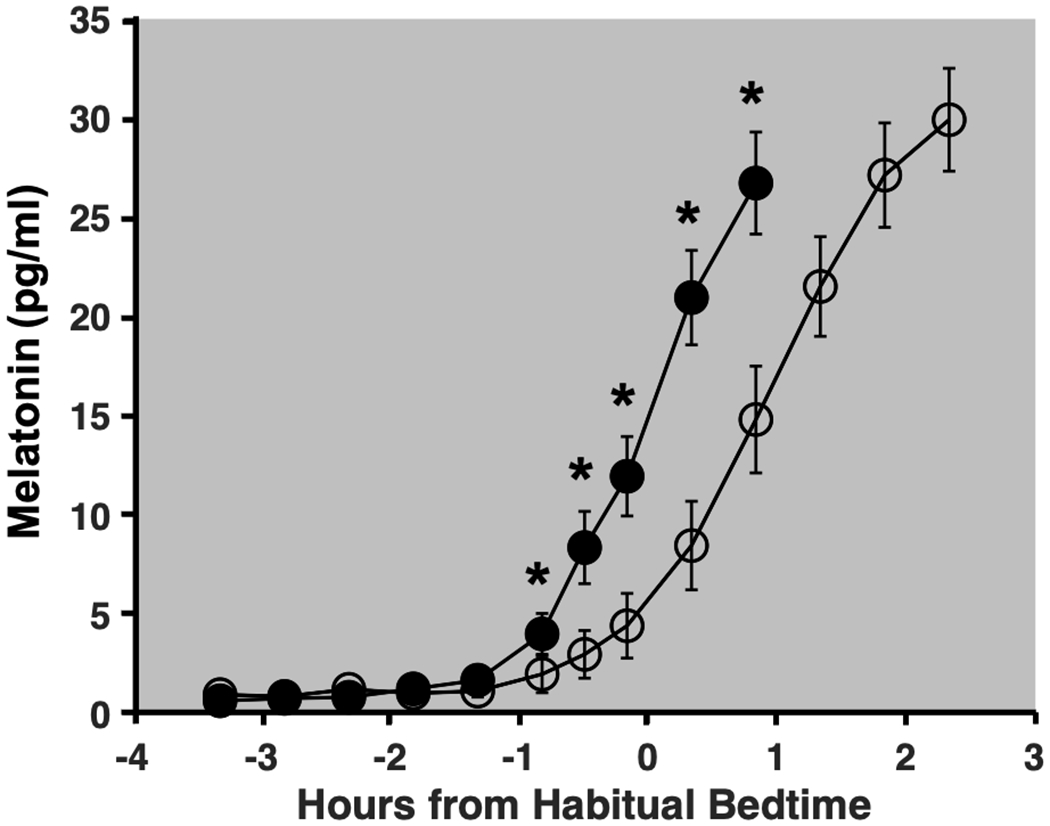
Closed circles represent saliva samples collected during the Baseline night (Day 8) and open circles represent those collected on the Final night of the study protocol (Day 10). Error bars denote standard error. Melatonin levels on Day 10 were significantly lower than at the same clock time on Day 8 at 50 min, 30 min, and 10 min before habitual bedtime, as well as 20 min and 50 min after habitual bedtime.
Fig 4: Circadian phase shift as a function of light intensity.
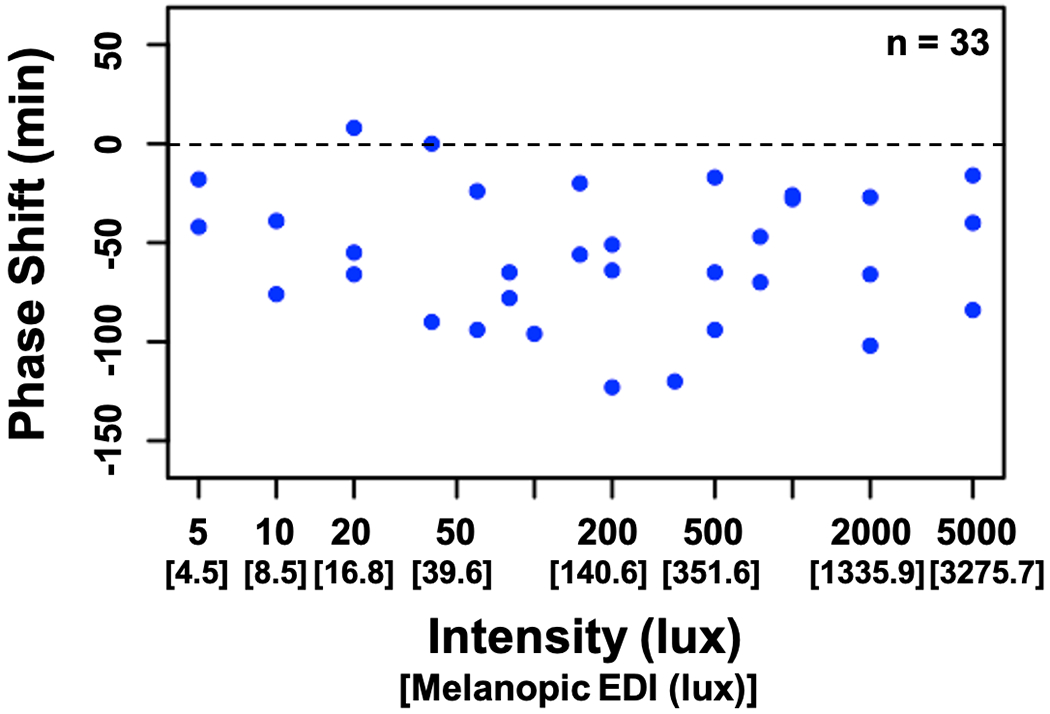
Negative numbers (below the dashed line) indicate a phase delay, whereas positive numbers (above the line) indicate a phase advance.
Fig 5: Melatonin profiles of 6 participants.
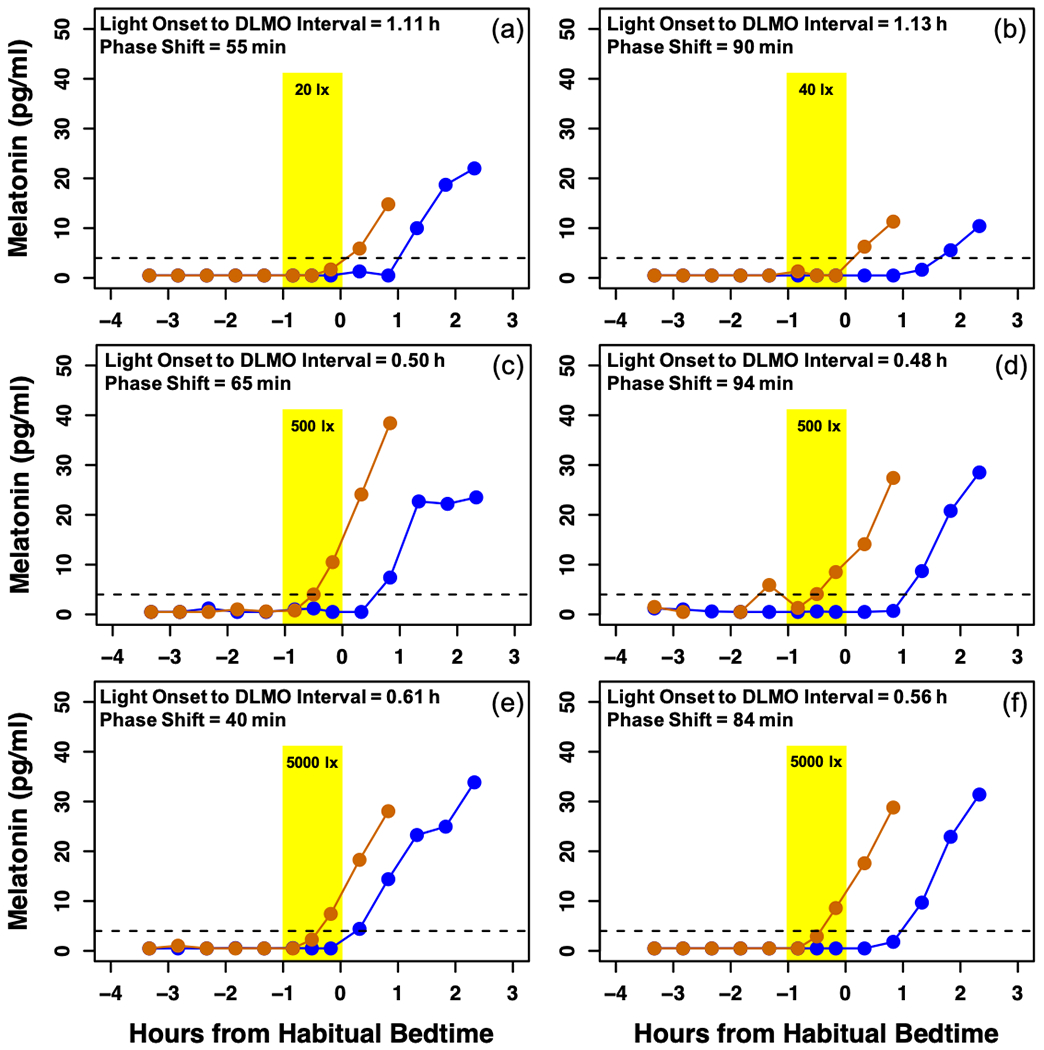
Orange lines represent melatonin on the baseline night and blue lines represent melatonin on the final night of the assessment. The dotted line at 4 pg/ml depicts the threshold for melatonin onset. The timing of the 1h light exposure is represented by the yellow shaded area. A and B depict the melatonin profiles of children who received low intensities of light at close circadian times. Similarly, participants C and D received a medium intensity of light, and participants E and F received a high intensity. In each pair, despite similarities in the circadian timing and intensity of the experimental light exposure, large individual differences in circadian phase shift were observed.
Data from 9 participants were excluded from the melatonin suppression analysis because the clock time of their baseline DLMO was more than 50 min after the clock time of the start of the light exposure (i.e., after the timing of the final saliva sample taken during the light exposure) (Hartstein et al., 2022). The percent melatonin suppression during the light exposure was correlated with the magnitude of phase shift observed the following evening (r = −0.73, p < 0.01; Fig 6). Greater acute melatonin suppression was associated with a greater subsequent phase delay.
Fig 6: Scatterplot illustrating association between acute percent melatonin suppression (Day 9) and subsequent phase shift (Day 10).
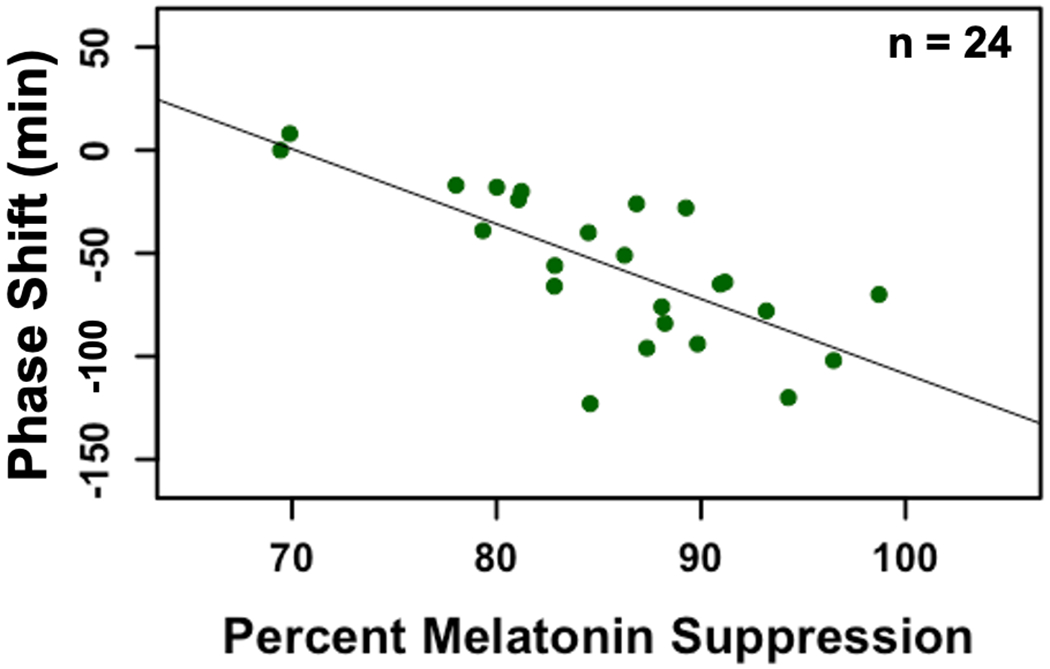
We observed a significant negative association between the magnitude of the melatonin suppression and phase shift (r = −0.73, p < 0.01).
Discussion
In this highly-controlled, randomized experimental study, 1 h of light exposure in the hour before bedtime resulted in significant delays in young children’s circadian timing across a wide range of light intensities (5 – 5,000 lux). Across all participants, the light exposure resulted in an average phase delay of 56 min, although large inter-individual variability was observed. Contrary to our hypothesis, no relationship between light intensity and the magnitude of the phase shift was observed. These data add to a growing literature suggesting that some young children may be highly sensitive to evening light, even at low intensities (Akacem et al., 2018; Hartstein et al., 2022).
In several instances, we observed large individual differences in response to similarly bright light exposures presented at the same circadian time (Fig 5). One contributing factor to these differences could be the length of an individual child’s circadian period (tau). The average circadian period in adolescents is consistent with that observed in adults (Crowley & Eastman, 2018); however, period length in young children is currently unknown. Under the dim-light conditions in the present study, melatonin onset in children with a longer circadian period would have drifted later, regardless of the experimental light exposure, than those with a shorter circadian period. It is possible, therefore, that the larger phase delays we observed in some children were due in part to having longer circadian periods. Despite controlling for each individual’s tau, one study with adolescents still observed large inter-individual variability in the phase-shifting response to light (Crowley & Eastman, 2017). Additionally, differences in photosensitivity have been reported in adults based on individual characteristics such as age, sex, chronotype, pupil size, light history, and genetic haplotypes (Chellappa, 2020). Such individual differences may also underlie some of the variability in our results. Given the challenges of conducting frequent repeated circadian assessments in young participants, future work should examine children’s circadian responses to light longitudinally in order to identify individual differences in tau and photosensitivity in the context of early childhood.
Previous research with adults employed much longer light exposures time-locked to circadian phase in order to establish illuminance response curves (Boivin, Duffy, Kronauer, & Czeisler, 1996; Duffy & Wright, 2005; Gooley et al., 2010; Zeitzer et al., 2000). In contrast to a light exposure of several hours long after participants’ scheduled bedtime (Gooley et al., 2010; Gronfier, Wright Jr, Kronauer, Jewett, & Czeisler, 2004; Zeitzer et al., 2000), the timing and length of the light exposure in the present study (1 h in the hour before bedtime) was chosen to reflect when young children are typically exposed to evening light, including frequent use of screen-based media devices before bed (Rideout & Robb, 2020). St. Hilaire and colleagues compared a phase response curve of a 1-h bright light exposure (~8,000 lux) to one previously measured in response to a 6.7-h exposure (St Hilaire et al., 2012). Their findings indicated that the 1-h exposure resulted in 40% of the phase shifting response elicited by the longer exposure, despite being only 15% of the duration, demonstrating that meaningful shifts in circadian timing can be elicited by a relatively short light exposure duration. Additionally, Crowley and Eastman established a phase response curve in adolescents (14.3 to 17.8 years) in response to an intermittent bright light exposure (5000 lux, four 20-min exposures) (Crowley & Eastman, 2017). Their results indicated that the maximal phase delay was −1.8 h, occurring 1.9 h after DLMO, and that a wide region of large delays (>1.0 h) spanned from 0.6 h before DLMO to 4.3 h after DLMO. In the present study, the midpoint of the 60-min light exposure occurred between 0.6 h before DLMO to 1.6 h after DLMO, within the region of large circadian delays observed in adolescents. Taken together, these previous findings suggest that substantial phase shifts could be expected given the timing and duration of the light exposure protocol used here.
A strong association between acute melatonin suppression and phase shift was observed, such that participants with greater melatonin suppression during the light exposure also had greater delays in circadian timing the following evening. This finding is somewhat surprising given the high levels and limited variability of melatonin suppression. Some prior work with adults has similarly demonstrated a linear correlation between melatonin suppression and circadian phase shift (Lockley, Brainard, & Czeisler, 2003). However, these outcomes have also been shown to be functionally independent (Rahman et al., 2018). Light-induced phase shifts occur throughout the 24-h day, regardless of the secretion of melatonin in both adults and adolescents (Crowley & Eastman, 2017; Jewett et al., 1997; Khalsa, Jewett, Cajochen, & Czeisler, 2003). Additionally, intermittent evening light exposures of varying intensities can produce different patterns of melatonin suppression and phase delay (Rahman et al., 2018). As this is the first study to date in which these outcomes were examined in young children, further research is needed to unpack this relationship and its possible implications for children’s circadian physiology.
Several limitations of these data should be noted. First, a strict set of eligibility criteria were employed, resulting in a population of only healthy, good-sleeping participants. The generalizability of these findings is therefore limited with respect to broader demographic and clinical populations. Secondly, the bedtimes of preschool-aged children are typically parent- rather than self-selected, which leads to large variability in habitual bedtime phase angle. As such, anchoring our light exposure to habitual bedtime resulted in light start occurring at different circadian phases for individual children. Although the variation and narrow bedtime phase angle we observed is consistent with our prior findings in this age group (Akacem et al., 2018; LeBourgeois, Carskadon, et al., 2013), the differences in the circadian timing of the light exposure could have played a role in our findings. In addition, the length of the circadian period in young children is currently unknown, and individual differences in intrinsic period could have led to variability in the phase shift response. Also, because we did not perform a 0-lux control condition for each participant, it is possible that drift in phase due to circadian period as a result of the dim-light conditions could have contributed to the magnitude of the phase shifts observed. Additionally, light-induced circadian phase delays are greater in adults after spending 3 days in dim light (11 lux) compared with 3 days in room light (90 lux) (Chang, Scheer, & Czeisler, 2011). Because children spent 27.5 h in dim light prior to the light exposure, they may have exhibited greater phase delays than would be observed following their typical light history. Finally, large inter-individual differences in phase shifts were observed in adolescents in response to the same light stimulus given at the same circadian time (Crowley & Eastman, 2017). Given the differences in circadian timing and intensity of our light exposure, we are not able to examine individual differences in young children’s photosensitivity and the underlying factors in the present dataset.
In summary, 1 h of light exposure in the hour before bedtime led to significant delays in circadian timing in preschool-aged children across a wide range of light intensities. These data suggest that the home evening lighting environment and its associated effects on the circadian timing system should be further examined as a contributor to late sleep onset and bedtime resistance in early childhood.
Acknowledgements
We are grateful to the children and families who participated in this research. We also thank the students and staff of the Sleep and Development Lab at the University of Colorado Boulder for their assistance in collecting these data.
Funding
This research was supported with funds from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01-HD087707), the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32- HL149646), The University of Colorado Boulder Undergraduate Research Opportunities Program, and the University of Colorado Boulder Biological Sciences Initiative Scholars Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
LDA and SRS have no financial or personal conflicts to declare. LEH reports receiving research support from the National Institutes of Health, outside the submitted work. CDB reports receiving research support from the National Institutes of Health, the National Science Foundation, and LumosTech, outside the submitted work. KPW reports research support/donated materials: DuPont Nutrition & Biosciences, Grain Processing Corporation, and Friesland Campina Innovation Centre and being a consultant to and/or receiving personal fees from Circadian Therapeutics, Inc., Circadian Biotherapies, Inc., Philips, Inc, and U.S. Army Medical Research and Materiel Command - Walter Reed Army Institute of Research, and receiving research support from the National Institutes of Health and the PAC-12 conference, outside the submitted work. MKL reports receiving travel funds from the Australian Research Council and research support from the National Institutes of Health, beyond the submitted work.
References
- Akacem LD, Wright KP Jr, LeBourgeois MK (2016) Bedtime and evening light exposure influence circadian timing in preschool-age children: A field study. Neurobiol Sleep Circadian Rhythms 1(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akacem LD, Wright KP Jr, LeBourgeois MK (2018) Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Res 6(5):e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramini AU and Hertzig ME (1983) Sleep and bedtime behavior in preschool-aged children. Pediatrics 71(2):153–158. [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA (1996) Dose-response relationships for resetting of human circadian clock by light. Nature 379(6565):540–542. [DOI] [PubMed] [Google Scholar]
- Bruni O, Reto FL, Miano S, Ottaviano S (2000) Daytime behavioral correlates of awakenings and bedtime resistance in preschool children. Suppl Clin Neurophysiol 53:358–361. [DOI] [PubMed] [Google Scholar]
- Burgess HJ and Eastman CI (2005) The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res 14(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R (1997) An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms 12(3):278–289. [DOI] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA (2011) The human circadian system adapts to prior photic history. J Physiol 589(Pt 5):1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL (2020) Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep 44(2):zsaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ and Eastman CI (2017) Human adolescent phase response curves to bright white light. J Biol Rhythms 32(4):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ and Eastman CI (2018) Free-running circadian period in adolescents and adults. Journal Sleep Res, 27(5):e12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon S and Arendt J (1994) Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett 167(1-2):191–194. [DOI] [PubMed] [Google Scholar]
- Duffy JF and Wright KP Jr (2005) Entrainment of the human circadian system by light. J Biol Rhythms 20(4):326–338. [DOI] [PubMed] [Google Scholar]
- Eto T, Ohashi M, Nagata K, Shin N, Motomura Y, Higuchi S (2021) Crystalline lens transmittance spectra and pupil sizes as factors affecting light-induced melatonin suppression in children and adults. Ophthalmic Physiol Opt 41(4):900–910. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW (2010) Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2(31):31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R (2005) Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol 33(2):157–163. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC, O’Connor TG, Plomin R (2004) Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. J Am Acad Child Adolesc Psychiatry 43(6):744–751. [DOI] [PubMed] [Google Scholar]
- Gregory AM and O’Connor TG (2002) Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry 41(8):964–971. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP Jr, Kronauer RE, Jewett ME, Czeisler CA (2004) Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab 287(1):E174–E181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstein LE, Behn CD, Akacem LD, Stack N, Wright KP Jr, LeBourgeois MK (2022) High sensitivity of melatonin suppression response to evening light in preschool-aged children. J Pineal Res 72(2):e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Nagafuchi Y, Lee SI, Harada T (2014) Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab 99(9):3298–3303. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA (1997) Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol Regul Integr Comp Physiol 273(5):R1800–R1809. [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X (2008) Sleep and obesity in preschool children. J Pediatr 154(6):814–818. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549(3):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Smith A, Weissbluth M, Binns HJ, Christoffel KK (1999) Sleep and behavior problems among preschoolers. J Dev Behav Pediatr 20(3):164–169. [DOI] [PubMed] [Google Scholar]
- LeBourgeois MK, Carskadon MA, Akacem LD, Simpkin CT, Wright KP Jr, Achermann P, Jenni OG (2013) Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythms 28(5):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois MK, Wright KP Jr, LeBourgeois HB, Jenni OG (2013) Dissonance between parent-selected bedtimes and young children’s circadian physiology influences nighttime settling difficulties. Mind Brain Educ 7(4):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang S, Huang Y, Chen K (2017) Sleep duration and obesity in children: A systematic review and meta-analysis of prospective cohort studies. J Paediatr Child Health 53(4): 378–385. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA (2003) High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88(9):4502–4505. [DOI] [PubMed] [Google Scholar]
- Maski KP and Kothare SV (2013) Sleep deprivation and neurobehavioral functioning in children. Int J Psychophysiol 89(2):259–264. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A (2006) Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 29(10):1263–1276. [PubMed] [Google Scholar]
- Owens J (2008) Classification and epidemiology of childhood sleep disorders. Prim Care 35(3):533–546, vii. [DOI] [PubMed] [Google Scholar]
- Rahman SA, St Hilaire MA, Gronfier C, Chang AM, Santhi N, Czeisler CA, Klerman EB, Lockley SW (2018) Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol 596(11):2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout V and Robb MB (2020) The Common Sense census: Media use by kids age zero to eight. Retrieved from San Francisco, CA: Common Sense Media. [Google Scholar]
- Simola P, Niskakangas M, Liukkonen K, Virkkula P, Pitkäranta A, Kirjavainen T, Aronen ET (2010) Sleep problems and daytime tiredness in Finnish preschool-aged children-a community survey. Child Care Hlth Dev 36(6):805–811. [DOI] [PubMed] [Google Scholar]
- Simpkin CT, Jenni OG, Carskadon MA, Wright KP Jr, Akacem LD, Garlo KG, LeBourgeois MK (2014) Chronotype is associated with the timing of the circadian clock and sleep in toddlers. J Sleep Res 23(4):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW (2012) Human phase response curve to a 1 h pulse of bright white light. J Physiol 590(13):3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikotzky L and Sadeh A (2001) Sleep patterns and sleep disruptions in kindergarten children. J Clin Child Psychol 30(4):581–591. [DOI] [PubMed] [Google Scholar]
- Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA (2005) Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20(2):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA (2000) Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526(3):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]


