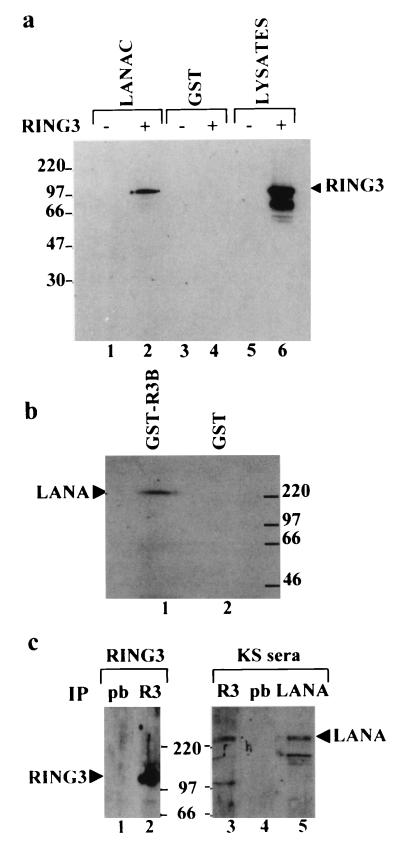
FIG. 1.
Interaction of RING3 and LANA. (a) RING3 binds to the C-terminal 212 amino acids of LANA. Lysates of uninfected Sf9 cells (lanes 1 and 3) or Sf9 cells infected with the recombinant RING3 baculovirus (lanes 2 and 4) were incubated with either GST-LANAC (lanes 1 and 2) or GST alone (lanes 3 and 4) immobilized on glutathione beads. Binding of RING3 was detected by Western blot analysis with an affinity-purified rabbit antibody raised against the R3B fusion protein (Fig. 3). (b) The complete LANA protein from the BCP-1 cell line binds to RING3. Cellular lysates of BCP-1 cells were incubated with a GST RING3 fusion protein (amino acids 554 to 754) (lane 1) or GST alone (lane 2). Binding of LANA was detected on Western blots with serum from a KS patient. (c) Detection of LANA-RING3 complexes in BCP-1 cells by coimmunoprecipitation. Lysates of BCP-1 cells were incubated with either the RING3 antibody (lanes 2 and 3) or prebleed (pb) rabbit serum (lanes 1 and 4). Immunoprecipitation (IP) with a rabbit antibody raised against the C-terminal LANA region was used as a positive control for the presence of LANA (lane 5). The protein-antibody complexes were isolated by using protein G beads and analyzed by Western blotting with either a rat anti-RING3 peptide antibody (a gift from K. Thorpe, Cambridge University, Cambridge, United Kingdom) (lanes 1 and 2) or human serum from a KS patient (lanes 3 to 5). The positions of the RING3 and LANA proteins are indicated. The sizes of the protein markers are shown in kilodaltons.