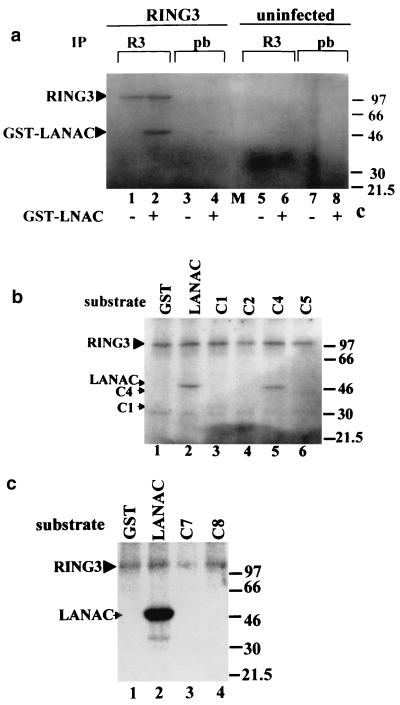
FIG. 4.
The C-terminal domain of LANA becomes phosphorylated in the presence of RING3. (a) Cellular lysates of SF9 cells infected with the RING3 baculovirus (lanes 1 to 4) or uninfected SF9 cells (lanes 5 to 8) were immunoprecipitated (IP) with either a rabbit antibody to RING3 (lanes 1, 2, 5, and 6) or prebleed (pb) rabbit serum (lanes 3, 4, 7, and 8). The immunoprecipitated proteins were added to an in vitro kinase assay mixture containing either 500 ng of GST-LANAC (lanes 2, 4, 6, and 8) or 500 ng of GST alone (lanes 1, 3, 5, and 7) as substrates and [γ-32P]ATP. Phosphorylated proteins were resolved by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. The positions of the phosphorylated RING3 and GST-LANAC proteins are indicated, and the sizes of the protein markers are indicated in kilodaltons on the right. (b) A 500-ng portion of each of the GST LANA deletion proteins C1 (lane 3), C2 (lane 4), C4 (lane 5), and C5 (lane 6) (Fig. 2) was used as a substrate in an in vitro kinase assay in the presence of the recombinant RING3 protein. GST (lane 1) or GST-LANAC (lane 2) were used as negative and positive controls, respectively. (c) A 500-ng portion of each of the GST-LANA deletion proteins C7 (lane 3) and C8 (lane 4) were used as substrates in an in vitro kinase assay.