Figure 2.
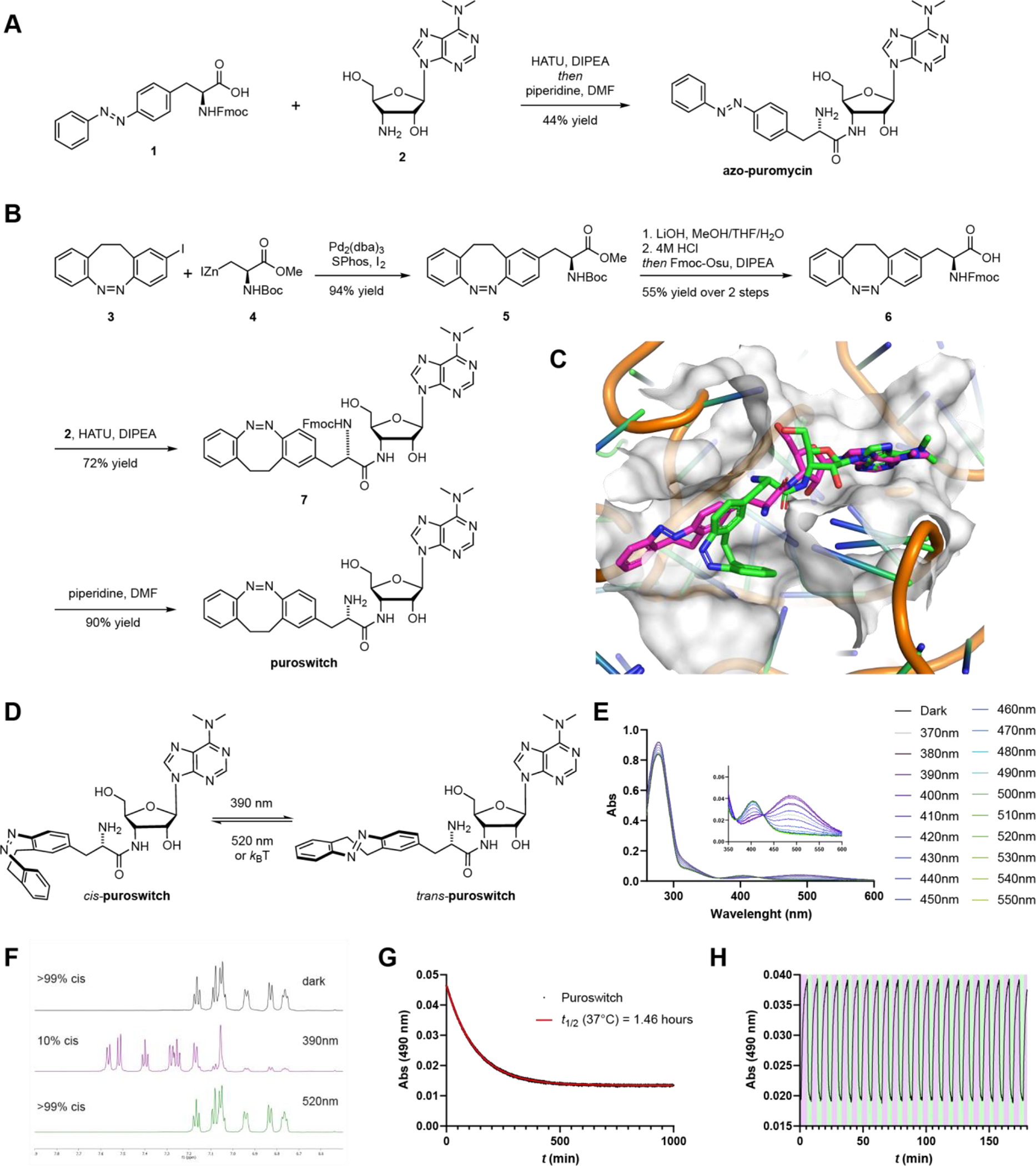
Design, synthesis and photophysical characterization of photoswitchable puromycin compounds. A) Synthesis of azo-puromycin. B) Synthesis of puroswitch. C) Docking of puroswitch in the A site of the ribosome. Best pose from the cis (green) and trans (purple) puroswitch isomers are shown. Model is derived from the structure of CC-puromycin bound to the A site of the 50S ribosomal subunit (PDB: 1Q82). D) Isomerization of cis-puroswitch to trans-puroswitch. E) UV-vis spectra of puroswitch (50 μM) in the dark and at different photostationary states in DMSO at r. t. F) 1H-NMR spectra of puroswitch with different PSS’ in DMSO at r.t. G) Thermal relaxation of puroswitch (50 μM) at 37°C in DMSO H) Reversible trans → cis isomerization of puroswitch (50 μM) at 390 (purple):520 (green) nm irradiation in DMSO at r.t.
