Abstract
Adenosine receptor (AR) suppresses inflammation and fibrosis by activating cyclic adenosine monophosphate (cAMP) signaling. We investigated whether altered AR expression contributes to the development of fibrotic diseases and whether A2AAR and A2BAR upregulation inhibits fibrotic responses. Primary human lung fibroblasts (HLFs) from normal (NHLFs) or patients with idiopathic pulmonary fibrosis (DHLF) were used for in vitro testing. Murine models of fibrotic liver or pulmonary disease were developed by injecting thioacetamide intraperitoneally, by feeding a high-fat diet, or by intratracheal instillation of bleomycin. Modafinil, which activates cAMP signaling via A2AAR and A2BAR, was administered orally. The protein amounts of A2AAR, A2BAR, and exchange protein directly activated by cAMP (Epac) were reduced, while collagen and α-smooth muscle actin (α-SMA) were elevated in DHLFs compared to NHLFs. In liver or lung tissue from murine models of fibrotic diseases, A2AAR and A2BAR were downregulated, but A1AR and A3AR were not. Epac amounts decreased, and amounts of collagen, α-SMA, KCa2.3, and KCa3.1 increased compared to the control. Modafinil restored the amounts of A2AAR, A2BAR, and Epac, and reduced collagen, α-SMA, KCa2.3, and KCa3.1 in murine models of fibrotic diseases. Transforming growth factor-β reduced the amounts of A2AAR, A2BAR, and Epac, and elevated collagen, α-SMA, KCa2.3, and KCa3.1 in NHLFs; however, these alterations were inhibited by modafinil. Our investigation revealed that A2AAR and A2BAR downregulation induced liver and lung fibrotic diseases while upregulation attenuated fibrotic responses, suggesting that A2AAR and A2BAR-upregulating agents, such as modafinil, may serve as novel therapies for fibrotic diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11302-023-09973-8.
Keywords: Fibrosis, Lung and liver, A2A and A2B adenosine receptors, Epac signalling
Introduction
Fibrosis is a normal healing response to injury and is associated with the accumulation of extracellular matrix (ECM) proteins and other materials in the tissue. Additionally, it is a pathological feature of most chronic inflammatory diseases afflicting various organs such as the liver and lungs [1]. When injury is repetitive or severe, fibrotic response is upregulated and continuously producing ECM material such as collagen (Col) which thereby accumulates in tissue, which can lead to disruption of tissue architecture—deteriorating the functions of organ systems. Fibrotic diseases are estimated to cause over 800,000 deaths each year worldwide [2], and the incidence of fibrotic diseases will increase further with an increase in the aged population [2–4].
Idiopathic pulmonary fibrosis (IPF), the most common type of pulmonary fibrosis, is a condition in which the lungs become injured diffusely and progressively in the parenchyma, and has a poor prognosis with a median survival of only two to three years [5]. The adjusted incidence and prevalence of IPF was estimated to be in the range of 0.09–1.30 and 0.33–4.51 per 10,000 persons, respectively [6]. Chronic inflammatory liver diseases are caused by infection with hepatitis B or C virus, fat accumulation, or chronic exposure to hepatotoxic agents such as alcohol. In the United States, non-alcoholic fatty liver disease occurs in 25–30% of the general population and can progress to nonalcoholic steatohepatitis [7]. Liver fibrosis occurs in most chronic inflammatory diseases. Despite the high prevalence and clinical importance of fibrotic diseases, their pathophysiology is yet to be elucidated, and anti-fibrotic therapeutics have not yet been developed.
Cyclic adenosine monophosphate (cAMP) signaling modulates inflammatory and fibrotic responses via an exchange protein activated by a cAMP (Epac)- and protein kinase A (PKA)-mediated pathway [8–11]. The intermediate conductance Ca2+-activated K+ channel KCa3.1 may play an important role in the modulation of inflammatory and fibrotic responses by cAMP. KCa3.1 regulates a variety of cellular processes involved in inflammatory and fibrotic processes, including cell activation, migration, and proliferation, by modulating Ca2+ signaling [12, 13]. KCa3.1 upregulation occurs in inflammatory and fibrotic responses and contributes to the development of fibrotic diseases. Conversely, KCa3.1 downregulation (or inhibition) attenuates inflammatory and fibrotic responses. Cyclic AMP downregulates KCa3.1 via an Epac-mediated pathway [12] and inhibits the KCa3.1 current through PKA-dependent phosphorylation of KCa3.1 [14]. Thus, cAMP-elevating agents such as modafinil (MF) [12, 14, 15] might modulate inflammation and fibrosis via Epac- and PKA-mediated pathways. MF stimulated cAMP production in various cells such as fibroblasts and osteoblasts [14, 16], and MF-induced signaling was inhibited by an A2A AR or A2B AR inhibitor [16], suggesting that A2A AR and A2B AR play an important role in MF-induced signaling.
The adenosine receptors (ARs; A1, A2A, A2B, and A3) are G-protein coupled receptors (GPCRs) that affect cellular cAMP levels [17]. A2AAR and A2BAR elevate cAMP levels via Gs-protein, whereas A1AR and A3AR decrease cAMP levels via Gi protein. A2AAR-null mice are more susceptible to bleomycin (BLM)-induced lung injury [18], suggesting that A2AAR play an important role in inhibiting pulmonary fibrosis in mice. A2BAR is expressed in most inflammatory cells and has both proinflammatory and anti-inflammatory effects [19]. It generates anti-inflammatory effects when coupled with protein Gs and proinflammatory effects when coupled with Gq protein [20]. The predominant AR in cardiac fibroblasts is the A2BAR, which modulates multiple processes in cardiac fibrosis [21]. Stimulation of A2BAR inhibited myofibroblast differentiation and Col synthesis by pro-fibrotic agonists, such as transforming growth factor β (TGFβ) in cardiac fibroblasts [21]. Thus, we examined whether reducing cAMP signaling by altering the expression of ARs contributes to the development of fibrotic diseases and whether the restoration of downregulated A2AAR and A2BAR could serve as a novel therapeutic approach for fibrotic diseases.
Materials and methods
Chemicals
(S)-Isomer of MF (MF-S) was synthesized by Cellion Biomed Inc. (Daejeon, South Korea), according to a published procedure [22]. Thioacetamide (TAA), bleomycin (BLM) and TGFβ were purchased from Sigma-Aldrich (St. Louis, MO). MRS 1754 (MRS), ZM 241,385 (ZM), and 5’-N-Ethylcarboxamidoadenosine (NECA) were from Tocris Bioscience (Minneapolis, MN). The other reagents used in the study were purchased from Sigma-Aldrich unless otherwise specified. MF was first dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO was 0.5% (diluted in distilled water).
Murine model of NASH and MF-S treatment
A choline-deficient, L-amino-acid-defined, high-fat diet with 0.1% methionine (CDAHFD; A06071302, Research Diets, New Brunswick, NJ, USA) was used to induce steatosis, steatohepatitis, and hepatic fibrosis in mice [23]. Six-week-old C57BL/6 male mice (18–22 g; n = 50; Oriental Bio, Seoul, Korea) were randomly divided into control and CDAHFD group and fed a standard laboratory murine diet and CDAHFD, respectively. The mice in these CDAHFD groups were fed a CDAHFD for 20 weeks.
Mice fed CDAHFD were randomly assigned to either vehicle- (CDAHFD group; n = 10) or MF-S-treated groups (CDAHFD + MF-S group; n = 30). MF-S was administered orally once daily, 5 times/week, and at four weeks after the start of CDAHFD for 16 weeks at doses of 10, 50, or 100 mg/kg (5–10/each dosage of MF-S). The control and CDAHFD groups were administered only the vehicle at the start of MF-S administration.
After 20 weeks, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight), and their livers and blood samples were collected for analysis.
Murine model of toxic hepatitis and MF-S treatment
TAA have been used to induce hepatitis and fibrosis. Six-week-old C57BL/6 male mice (n = 26) were randomly subdivided into two groups: control mice (n = 8) that received the vehicle for 16 weeks, and mice in the TAA-induced liver disease group (n = 35) that received TAA for 16 weeks.
Mice in the TAA-induced liver disease group were randomly assigned to either the vehicle- (TAA group; n = 13) or MF-S-treated groups (TAA + MF-S group; n = 25). MF-S was administered orally once daily and 5 times/week from 8 weeks after the start of TAA for 8 weeks at dosages of 10, 50, or 100 mg/kg (7–10/each dosage of MF-S). The control and CDAHFD groups were administered the vehicle at the start of MF-S administration.
TAA (100 mg/kg) was injected intraperitoneally three times per week, whereas MF-S was administered orally five times per week. Three mice in the TAA group were sacrificed eight weeks after TAA administration to confirm TAA-induced liver inflammation and fibrosis, while the remaining mice were sacrificed 16 weeks after TAA administration. Mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight), and their livers and blood samples were collected for analysis.
Murine model of pulmonary fibrosis and MF-S treatment
BLM has been used to induce pulmonary inflammation and fibrosis. Six-week-old C57BL/6 male mice (n = 50) were randomly subdivided into three groups: control mice (n = 10) that received the vehicle; mice in the BLM-induced pulmonary disease group (BLM group, n = 10) that received BLM (1.5 units/kg); and mice in the treatment groups (BLM + MF-S) that received BLM + MF-S for three weeks.
BLM (1.5 units/kg) was administered intratracheally once. MF-S (10, 50, or 100 mg/kg) was orally administered five times per week (n = 10/each per dosage) starting from one week after BLM instillation. The control and BLM groups were administered only the vehicle at the start of MF administration.
Four weeks after BLM instillation, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight); the lungs and blood samples were collected for analysis.
Histopathology and immunohistochemistry
The liver and lung specimens were fixed overnight in 10% formalin solution, then dehydrated, embedded in paraffin, and cut into 4-µm sections. Sections were stained with hematoxylin (Carrazzi’s Hematoxylin Solution, FUJIFILM Wako Pure Chemical Corporation) and eosin (ClearView™ Eosin, BBC Biochemical) (H&E), Masson’s trichrome (Masson’s Trichrome Stain Kit, Artisan, AR173, Agilent DAKO, Santa Clara, CA, USA), and Picro Sirius red stain kit (ab150681, Abcam, Cambridge, UK). For immunohistochemical analysis, sections were incubated with primary antibodies against Collagen 1(Col1, NBP1-77458, Novus Biologicals), α-smooth muscle actin (α-SMA, ab7817, Abcam), CD45 (ab10558, Abcam) and reticulin (Artisan Reticulin/No counterst Stain Kit, AR18211-2, Agilent Technologies, Santa Clara, CA) for 1 h at 37˚C, then processed by an indirect immune-peroxidase technique using a commercial kit (ImmPACT DAB Peroxidase Substrate Kit, SK-4105, Vector Laboratories). The slides were examined with a BX50 microscope (Olympus, Tokyo, Japan) and analyzed with the iSolution DT software (IMT i-Solution, Vancouver, BC, Canada).
Cell culture and treatment of cells with reagents
Primary human lung fibroblasts from a normal adult (NHLFs, CC-2512) and primary human lung fibroblasts from a patient with IPF (DHLFs, CC-7231) were purchased from Lonza (Basel, Switzerland) and cultured at 37 °C in Fibroblast growth medium 2 (CC-3131; Lonza).
All cells were maintained at 37 °C in a humidified condition under 5% CO2. Media were changed twice weekly and cultures were passaged at a dilution of 1:2 weekly. The medium was then removed and replaced with fresh medium; the cells were maintained for the time periods indicated.
Quantitative polymerase chain reaction (PCR)
RNA isolation was performed using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and RNA was then reverse-transcribed using a high-capacity complementary DNA archive kit (Applied Biosystems, Foster City, CA, USA). PCR was performed on an ABI 7000 sequence detection system (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems). Primer sets (Table 1) used for real-time PCR were designed using Primer3 (http://frodo.wi.mit.edu/primer3).
Table 1.
PCR Primers designed to amplify subtypes of adenylate cyclase (AC)
| primer | Sense | Anti-sense |
|---|---|---|
| AC2 | 5’-GAG CGG AGA GTA CTG GAT CG-3’ | 5’-GCT TCT TTT CCT TGG GGT TC-3’ |
| AC3 | 5’- GGC TTC AGA GAC AAG GAA CG -3’ | 5’- TGG AGG GAT TGT TTC CTC TG -3’ |
| AC9 | 5’- TCT TGA GCA AGC CGG ACT AT -3’ | 5’- CGA ACT CGA AGA GGA TAC GC -3’ |
| AC10 | 5’- AAA AAC CTC GAC CAC CAC AG -3’ | 5’- TTT CTG CCC CTC AGA TAT GG -3’ |
| GAPDH | 5’-CTC CCA CTC TTC CAC CTT CG-3’ | 5’-TAG GGC CTC TCT TGC TCA GT-3’ |
Western blotting analysis
Tissue or cell extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 7.5–12% gels and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with Tris-buffered saline with 0.1% Tween-20 containing 5% bovine serum albumin at room temperature. The membranes were immunoblotted using antibodies against A1AR (ab151523, Abcam), A2AAR (ab3461, Abcam), A2BAR (ab229671, Abcam) and A3AR (ab197350, Abcam), KCa3.1 (sc-365,265, Santa Cruz Biotechnology, Dallas, TX, USA), KCa2.3 (sc28621, Santa Cruz Biotechnology), Col1 (#66,948, Cell Signaling Technology Inc., Danvers, MA, USA), α-SMA (ab7817, Abcam), Epac1 (sc-28,366, Santa Cruz Biotechnology), and Epac2 (sc28326f, Santa Cruz Biotechnology), and bands were visualized by chemiluminescence. Especially, size validation for anti- A2AAR antibody was accomplished by antigen-peptide (Suppl. Figure 1). Data processing was performed using a LAS-3000 luminescent image analyzer and IMAGE GAUSE software (Fuji Film, Tokyo, Japan).
Procollagen type1 N-terminal propeptide (P1NP) measurement
The P1NP levels were measured from mice sera using a P1NP enzyme-linked immunosorbent assay kit (Mouse P1NP Elisa Kit, #MBS2500076; MyBioSource, San Diego, CA, USA). The experimental procedure was performed according to the manufacturer’s instructions.
Hydroxyproline colorimetric assay
Measurement of hydroxyproline in liver tissues was performed by manufacturer’s instruction.
(Hydroxyproline Assay Kit, MAK008, Sigma Aldrich). Briefly, tissues were homogenized and hydrolyzed for 3 h by adding concentrated hydroxychloride. Each hydrolyzed sample was transferred in a 96-well plate and dried under vacuum. After that, hydroxyproline concentration was determined by the reaction of oxidized hydroxyproline with 4-(dimethylamino)benzaldehyde, which results is proportional to the hydroxyproline content.
Transfection
Small interfering RNA (siRNA) against A2AAR (sc39850), A2BAR (sc29642) or scrambled was purchased by Santa Cruz Biotechnologies. NHLFs were transiently transfected for 24 h with the siRNA (100 pM) using siRNA transfection reagent (sc29528, Santa Cruz Biotechnology).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) values. To examine the statistical significance among groups, one-way analysis of variance (ANOVA) with Bonferroni’s post hoc or two-tailed Student t-test was employed. Values with P < 0.05 were considered to be statistically significant.
Results
The expression of ARs is altered in hepatic and pulmonary fibrotic diseases
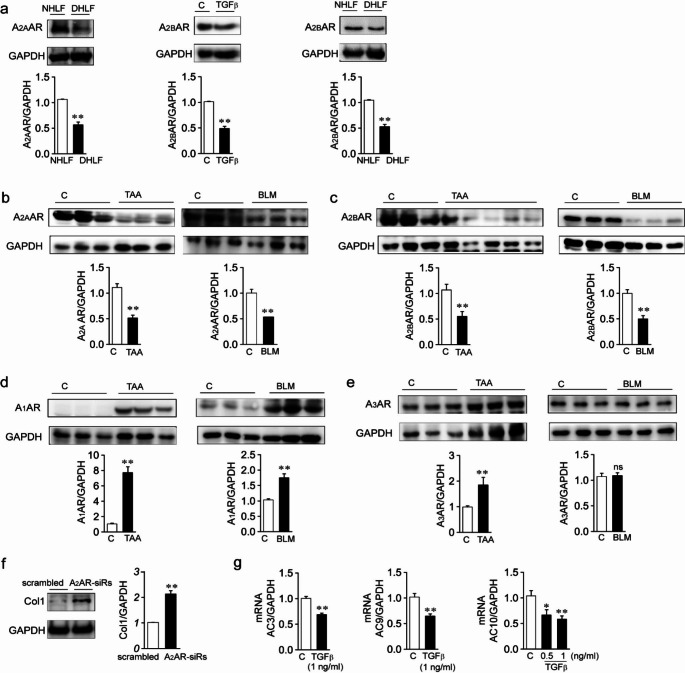
We compared the expression amounts of A2AAR and A2BAR between NHLFs and DHLFs. Protein amounts of A2AAR and A2BAR were significantly reduced in DHLFs compared to those in NHLFs (Fig. 1a, left and right panels). We then examined whether TGFβ, which plays a critical role in inflammation and fibrosis, affected the expression amounts of A2AAR and A2BAR in NHLFs. When NHLFs were treated with TGFβ for 24 h, the expression amounts of A2AAR (Fig. 5d, left panel) and A2BAR (Fig. 1a, middle panel) were significantly decreased compared to those in the control. We then examined whether alterations in the expression amounts of ARs occurred in murine models of fibrotic diseases (Fig. 1b-e). Protein amounts of A2AAR were decreased in liver tissue samples from TAA-induced liver fibrosis mice (Fig. 1b, left panel) or lung tissue samples from BLM-induced lung fibrosis mice (Fig. 1b, right panel) compared to the control, and protein amounts of A2BAR were decreased in liver tissue samples from TAA-induced liver disease mice (Fig. 1c, left panel) or lung tissue from BLM-induced lung fibrosis mice (Fig. 1c, right panel) compared to the control. A decrease in A2BAR amounts was also observed in liver tissue from CDAHFD-induced liver disease mice (Fig. 5c, left panel). In contrast, the protein amounts of A1AR and A3AR were not reduced in murine models of fibrotic diseases (Fig. 1d and e). A1AR amounts were significantly elevated in liver tissues from TAA-induced liver disease and lung tissues from BLM-induced lung disease compared to those in the control (Fig. 1d). A3AR amounts were significantly elevated in liver tissues from TAA-induced liver disease mice (Fig. 1e, left panel) and were not altered in lung tissues from BLM-induced lung disease mice (Fig. 1e, right panel). Then we examined whether knockdown of A2AAR and A2BAR affected Col1 expression in NHLFs. Col1 amounts were significantly increased by treatment with siRNA against A2AAR + siRNA against A2BAR (A2AR-siRs) (Fig. 1f). As ARs are coupled to ACs via G proteins, and thereby affect cAMP levels, we examined the mRNA levels of ACs. Messenger RNA levels of ACs were reduced in the TGFβ-treated NHLFs (Fig. 1g) and in lung tissue from BLM-induced lung fibrosis (Suppl. Figure 2) compared to the controls.
Fig. 1.
Expression amounts of ARs and ACs are altered in fibrotic diseases
Protein amounts of ARs (a-e) and Col (f) were measured by western blotting. a Expression amounts of A2AAR and A2BAR in NHLFs, DHLFs, and TGFβ (2ng/ml)-treated NHLFs. The blots shown are representative of three to five experiments performed using three to five different cultures. b Expression amounts of A2AAR in liver and lung tissues from wild-type and murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis. c Expression amounts of A2BAR in liver and lung tissues from wild-type and murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis. d and e Expression levels of A1AR (d) and A3AR (e) in the liver and lung tissues from wild-type and murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis, respectively. f Change in Col1 content of NHLFs transfected with scrambled siRNA or A2R-siRs. g Messenger RNA levels of ACs in NHLFs and TGFβ-treated NHLFs. NHLFs were treated with TGFβ for 24 h, and mRNA levels of ACs were measured by RT-PCR. Three to seven tissues per mice group were employed. Each experiment was repeated three times independently. Data are presented as the mean ± SEM values. *P < 0.05, **P < 0.01 vs. NHLFs or control
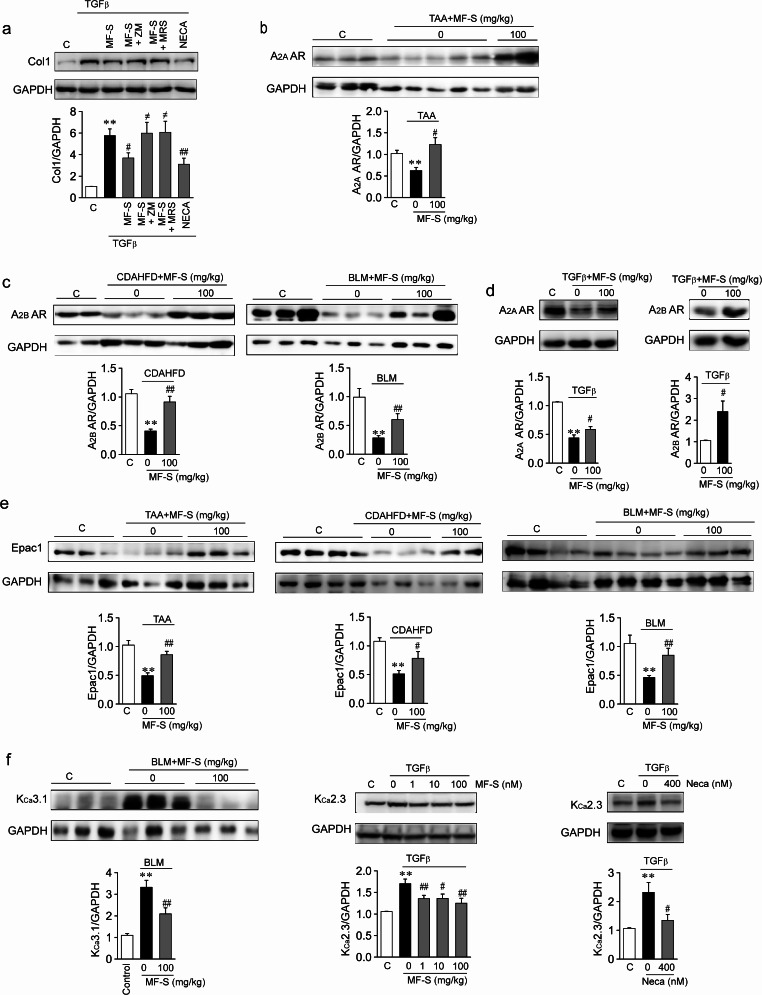
Fig. 5.
MF-S augments cAMP signaling by upregulating A2A AR and A2B AR
a Anti-fibrotic effects of MF were inhibited by an inhibitor of A2AAR or A2BAR. b and c Reduced A2AAR (b) and A2BAR (c) amounts in murine models of fibrotic diseases were restored after treatment with MF-S. d NHLFs were treated with TGFβ or TGFβ + MF over 24 h. TGFβ (2ng/ml) reduced expression amounts of A2AAR (d, left panel) and A2BAR (Fig. 1a, right panel). The A2AAR and A2BAR amounts were restored after MF-S treatment. e Downregulated Epac1 were restored by treatment with MF in murine models of fibrotic diseases. f Expression amounts of KCa3.1 in liver tissues from BLM-induced lung fibrosis (left panel) or KCa2.3 in NHLFs (middle and right panels). TGFβ (10ng/ml) elevated KCa2.3 levels, and this elevation was reversed by MF or NECA. d-f Blots shown are representative of the three to five experiments performed with three to five different cultures. Three to ten tissues per mice group were employed. Each experiment was repeated three to seven times independently. Data are presented as mean ± SEM values. **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. TAA-induced liver disease model or BLM-induced lung disease model
Cyclic AMP signaling is reduced in hepatic and pulmonary fibrotic diseases
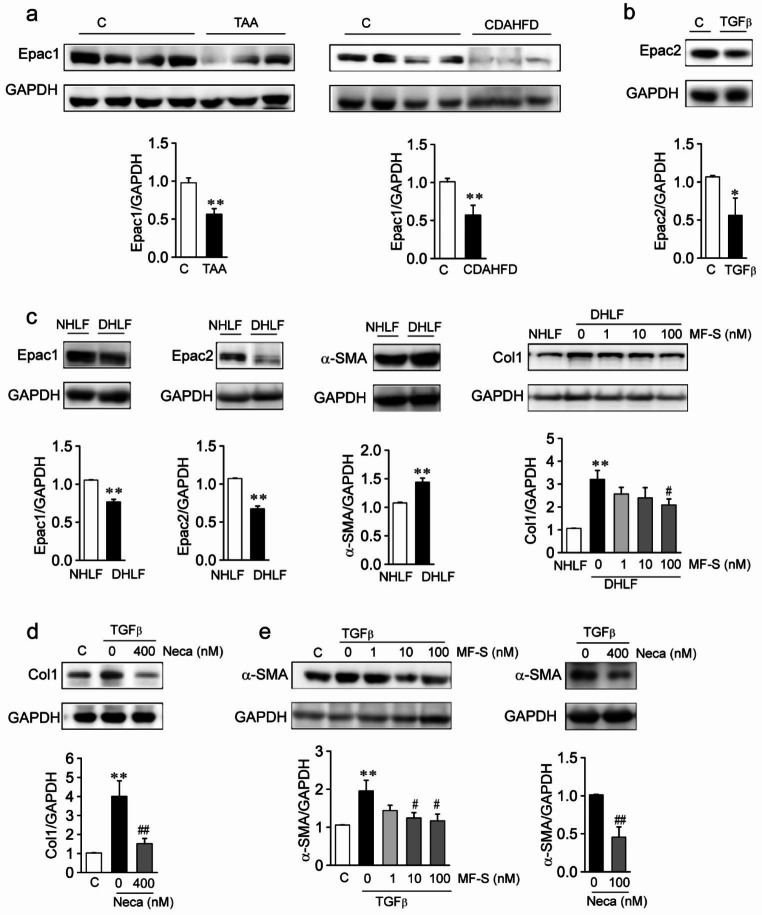
Reduction in the expression amounts of A2AAR, A2BAR, and ACs suggests that cAMP signaling is attenuated in fibrotic diseases. Thus, we compared Epac amounts in liver tissue from murine models of fibrotic liver diseases and found that Epac1 amounts were significantly decreased in the liver tissue from mouse models of TAA-induced and CDAHFD-induced liver fibrosis compared to the control (Fig. 2a). Since we have shown that TGFβ reduces the protein amounts of Epac1 in human-origin HSCs [12], we wished to examine whether TGFβ reduces the protein amounts of Epac2 (Fig. 2b). Epac2 amounts were found to be significantly reduced in TGFβ-treated NHLFs compared to the control. We then compared the expression amounts of Epacs, Col1, and α-SMA in NHLFs and DHLFs (Fig. 2c). Compared to NHLFs, Epac1 and Epac2 amounts were significantly reduced, and the amounts of Col1 and α-SMA were significantly elevated in DHLFs. Elevated amounts of α-SMA and Col1 in DHLFs suggest that DHLFs are activated fibroblasts. MF [12, 14, 16] or the pan AR activator NECA elevates cAMP levels, thereby activating Epac. We examined whether Epac activation using MF-S or NECA inhibited the fibrotic process in DHLFs and NHLFs. MF-S reduced Col1 amounts in DHLFs in a concentration-dependent manner (Fig. 2c, right panel). In addition, NHLFs were treated with TGFβ, TGFβ + NECA, or TGFβ + MF-S for 24 h. The amounts of Col and α-SMA were significantly elevated by TGFβ, while NECA or MF-S treatment significantly reduced these amounts (Fig. 2d and e).
Fig. 2.
cAMP signaling is reduced in fibrotic diseases
a Expression amounts of Epac1 in liver tissues from wild-type and murine models of liver fibrosis. Epac1 was significantly downregulated in liver fibrosis models compared to the control. Three to eight tissues per mice group were employed. Each experiment was repeated three times independently. b Expression amounts of Epac2 in NHLFs. Treatment with TGFβ (10 ng/ml) for 24 h reduced Epac2 levels. c Expression amounts of Epac1, Epac2, α-SMA, and Col1 in NHLFs and DHLFs. Epac1 and Epac2 amounts were significantly reduced, and α-SMA and Col1 amounts were significantly elevated in DHLFs compared to the NHLFs. d and e Expression amounts of Col1 (d) and α-SMA (e) in NHLFs. TGFβ (10 ng/ml) augmented expression amounts of Col1 and α-SMA. Augmented amounts of Col1 and α-SMA were reversed using cAMP-elevating agents: NECA (100 nM) or MF-S. b-e Blots shown are representative of the three to five experiments performed using three to five different cultures. Data are presented as mean ± SEM values. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. DHLFs or TGFβ-treated NHLFs.
These results suggest that cAMP signaling is reduced in murine models of fibrotic disease and that fibrotic progress is inhibited via activation of the cAMP/Epac pathway.
Therapeutic treatment with MF inhibits fibrotic progress in murine models
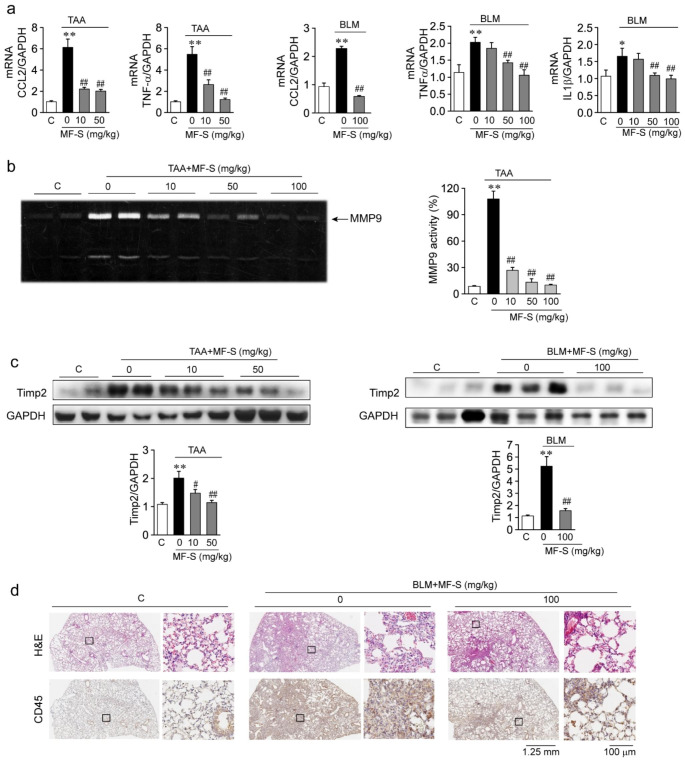
Prophylactic treatment with MF inhibited inflammatory and fibrotic responses in liver tissues from murine models of TAA-induced liver diseases, and MF-S may be a better alternative to (R)-isomer of MF (MF-R) as an anti-inflammatory and anti-fibrotic agent [12]. Thus, we examined whether MF-S as a therapeutic agent inhibits inflammatory responses in murine models of TAA-induced liver fibrosis and BLM-induced pulmonary fibrosis. Compared to the control, mRNA levels of CCL2, tumor necrosis factor-α (TNFα), and interleukin-1α (IL1α) were markedly elevated in liver or lung tissue samples from mice with TAA-induced liver disease or BLM-induced pulmonary disease, respectively, and significantly reduced in mice treated with MF-S (Fig. 3a). Matrix metalloproteinase-9 (MMP9) activity was significantly enhanced in liver tissue from mice with TAA-induced liver disease; however, activity was significantly reduced in a dose-dependent manner after treating with MF-S (Fig. 3b). In addition, compared with those from the control group, protein amounts of tissue inhibitor of metalloproteinase 2 (TIMP2) were significantly elevated in liver and lung tissues from mice with TAA-induced liver disease and BLM-induced lung disease, respectively, but were significantly reduced after treatment with MF-S (Fig. 3c). H&E staining of lung tissue sections was performed to observe histopathological changes occurring in the murine models. Compared with the control, cell infiltration was observed in the lung tissue of mice with BLM-induced pulmonary disease (Fig. 3d, upper panel). When BLM-induced pulmonary disease mice were treated with MF-S, cell infiltration was markedly reduced. We then examined CD45 amounts in lung tissue collected from lung disease murine models (Fig. 3d, lower panel). CD45 is expressed in almost all immune cells, such as T cells, B cells, and macrophages, to confirm whether the infiltrated cells are immune cells. CD45-positive cells were markedly increased in the lung tissue from mice with BLM-induced lung disease compared with the control. When BLM-induced pulmonary disease mice were treated with MF-S, CD45-positive cells were markedly decreased in the lung tissue samples. These results suggest that the inflammatory response activated in mice with fibrotic diseases in the liver or lung was suppressed by the MF treatment.
Fig. 3.
MF-S inhibits inflammatory responses in fibrotic diseases
a Messenger RNA levels of CCL2. TNF-α, and IL1β in liver or lung tissues from murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis. b MMP9 activity in liver tissues from murine models of TAA-induced liver fibrosis. c Protein amounts of TIMP2 in liver or lung tissues from murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis. d Representative images of H&E and CD45 staining in lung tissues from mice with BLM-induced pulmonary fibrosis. Enlargements of black boxes (solid line) were shown in right panels, respectively. Three to ten tissues per mice group were employed. Each experiment was repeated three times independently. Data are presented as mean ± SEM values. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. BLM-induced lung disease model
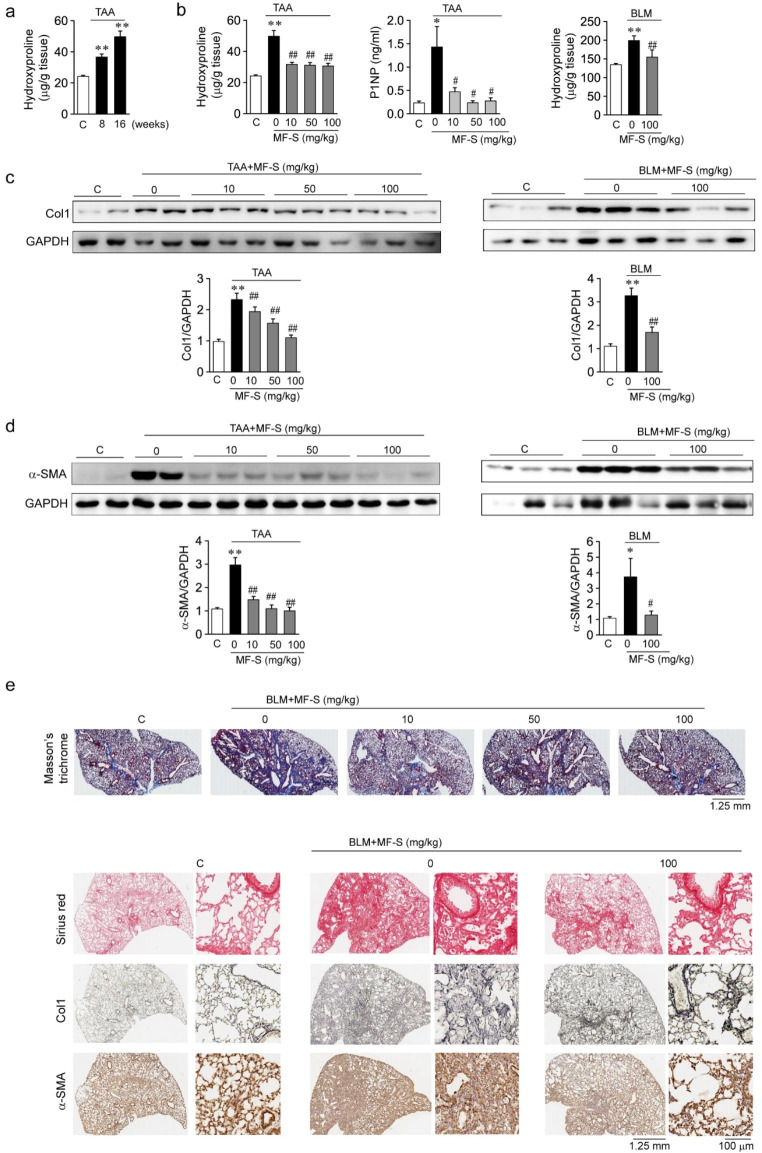
We then examined whether MF-S as a therapeutic intervention inhibits fibrotic responses in murine models of TAA-induced liver fibrosis and BLM-induced pulmonary fibrosis. Hydroxyproline levels in the mice liver tissue increased after TAA treatment. After a 16-week TAA treatment, hydroxyproline levels were significantly higher than those after an 8-week treatment (Fig. 4a), which suggests that the 16-week TAA treatment worsened liver fibrosis compared to the 8-week treatment. Tissue levels of hydroxyproline and serum levels of the Col1 metabolites P1NP increased in TAA-induced liver disease and BLM-induced pulmonary disease, and MF-S reduced the levels of hydroxyproline and P1NP in a dose-dependent manner (Fig. 4b). Protein amounts of Col1α (Fig. 4c) and α-SMA (Fig. 4d) were also elevated in liver and lung tissues from murine models of TAA-induced liver fibrosis and BLM-induced pulmonary fibrosis, respectively; MF-S treatment reduced Col1α and α-SMA amounts in murine models. We then performed immunostaining of tissue samples from murine models (Fig. 4e). Col1α was stained blue with Masson’s trichrome or red with Sinus red, and reticulin and α-SMA were stained brown. Compared with the control group, the intensity of the blue or red color of Col1α and brown color of reticulin and α-SMA were markedly higher in tissues from mice with BLM-induced lung disease. When mice with fibrotic diseases were treated with MF-S, the Colα and α-SMA amounts were markedly reduced. These results suggest that the fibrotic processes that cause the progression of liver and lung diseases in murine models were suppressed by MF treatment.
Fig. 4.
MF-S inhibits fibrotic responses in murine models of fibrotic diseases
a and b Hydroxyproline or P1NP levels in murine models of TAA-induced liver fibrosis or BLM-induced lung fibrosis. a Hydroxyproline levels in liver tissue from mice treated with TAA for 8 or 16 weeks. b Hydroxyproline or P1NP levels were elevated in models of fibrotic diseases, and elevated levels of hydroxyproline or P1NP were significantly reversed after MF-S treatment. c and d Expression amounts of Col1 (c) or α-SMA (d) in TAA-induced liver fibrosis and BLM-induced lung fibrosis murine models. e Representative images of Masson’s trichrome, Sirus red, reticulin, and α-SMA staining in lung tissues from mice with BLM-induced pulmonary fibrosis. Enlargements of black boxes (solid line) were shown in right panels. Three to ten tissues per mice group were employed. Each experiment was repeated three to five times independently. Data are presented as mean ± SEM values. *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. BLM-induced lung disease model
MF activates cAMP signaling via upregulating A2AAR and A2BAR
We examined whether cAMP signaling by MF was blocked by pharmacological inhibition of A2AAR and A2BAR [16]. ZM and MRS are high affinity antagonists of A2AAR and A2BAR, respectively. NHLFs were exposed to MF-S (100 nM), MF-S + ZM (10 nM), or MF-S + MRS (10 nM for 24 h, and expression amounts of Epac1 were examined (Suppl. Figure 3). MF-S increased Epac1 amounts, and the increased Epac1 by MF-S were reversed by ZM or MRS. Then, we examined whether anti-fibrotic effects of MF were mediated via A2AAR and A2BAR (Fig. 5a). NHLFs were exposed to TGFβ, TGFβ + MF-S, TGFβ + MF-S + ZM, TGFβ + MF-S + MRS, or NECA for 24 h. Col1 amounts were markedly increased by treatment with TGFβ in NHLFs whereas increased Col1 amounts by TGFβ was significantly reduced by MF-S or NECA treatment. The inhibitor of either receptor abrogated MF-induced reduction in Col amounts, suggesting that A2A AR and A2B AR are involved in anti-fibrotic effects of MF.
We examined whether MF-S treatment affects the expression amounts of A2AAR and A2BAR in lung and liver tissue from murine models of fibrotic diseases and in TGFβ-treated NHLFs. MF-S treatment restored the protein amounts of A2AAR in TAA-induced liver fibrosis (Fig. 5b) and A2BAR in the mouse models of CDAHFD-induced liver fibrosis and BLM-induced lung fibrosis (Fig. 5c). In addition, when NHLFs were treated with TGFβ over 24 h, the protein amounts of A2AAR (Fig. 5d, left panel) and A2BAR (Fig. 1a, right panel) were reduced. This TGFβ-induced reduction in the amounts of A2AAR and A2BAR was observed to be blocked by MF-S (Fig. 5d). We then examined mRNA levels of ACs in murine models of BLM-induced pulmonary fibrosis. The mRNA levels of ACs were reduced in BLM-induced lung fibrosis compared to the control, and MF-S blocked the reduction in ACs (Suppl. Figure 2). Since these results suggest that cAMP signaling in murine models of fibrotic diseases is restored after treatment with MF-S, we examined Epac1 amounts in murine models of fibrotic diseases. Epac1 amounts were reduced in liver and lung tissues from murine models of fibrous diseases compared to the control and were significantly elevated in those from MF-S-treated murine models (Fig. 5e). Since an Epac-mediated pathway modulated the expression amounts of KCa2.3 and KCa3.1, in murine models of liver fibrosis and HSCs [12], we examined whether similar changes in KCa2.3 and KCa3.1 amounts occurred in lung tissue from murine models of pulmonary fibrosis and TGFβ-treated NHLFs. KCa3.1 amounts were elevated in lung tissue from murine models of pulmonary fibrosis compared to the control and were significantly reduced in samples from MF-S-treated fibrosis models compared to those from BLM-induced lung fibrosis (Fig. 5f, left panel). In addition, KCa2.3 amounts were elevated by TGFβ in NHLFs; elevated KCa2.3 was then reversed by MF-S in a concentration-dependent manner (Fig. 5f, middle panel), or by NECA (Fig. 5f, right panel). These results suggest that A2A AR and A2B AR upregulation inhibits the fibrotic process by activating the cAMP pathway.
Discussion
This is the first study to demonstrate that downregulation of A2AAR and A2BAR contributes to the development of fibrotic diseases, and that fibrotic progression can be inhibited by upregulating A2AAR and A2BAR. Downregulation of A2AAR and A2BAR and a reduction in Epac amounts were observed in murine models of fibrotic liver and lung diseases, suggesting that downregulation of A2AAR and A2BAR contribute to the development of fibrotic diseases in the liver and lung by attenuating cAMP signaling. MF treatment upregulated A2AAR and A2BAR, elevated Epac amounts, and inhibited inflammatory and fibrotic responses in murine models of fibrotic diseases, suggesting that the upregulation of A2AAR and A2BAR inhibits inflammatory and fibrotic processes by enhancing cAMP signaling. Therefore, A2AAR and A2BAR may be therapeutic targets against inflammatory and fibrotic diseases, including liver and lung fibrosis, and A2AAR and A2BAR-upregulating agents, such as MF, are potential therapeutic agents for fibrotic diseases.
Cyclic AMP modulates inflammatory and fibrotic responses via Epac- and PKA-mediated pathways. Cyclic AMP/Epac signaling exerts anti-fibrotic actions by inhibiting epithelial–mesenchymal transformation and ECM formation. In contrast, pro-fibrotic agents, such as TGFβ and angiotensin II, downregulate Epac, thereby promoting fibrotic responses [9]. In addition, cAMP/PKA signaling can inhibit inflammatory and fibrotic responses. Continuously elevated cAMP levels inhibit immune cell functions, such as the proliferation and activation of T and B cells [8], and PKA signaling may be involved in anti-inflammatory and anti-fibrotic pathways [8, 24]. Downregulation of KCa2.3 and KCa3.1 may play a critical role in the anti-inflammatory and anti-fibrotic effects of cAMP. Pro-inflammatory cytokines and pro-fibrotic growth factors, such as TGFβ [12] and VEGF [25], upregulate KCa3.1 and KCa2.3, and pharmacological inhibition or knockdown of KCa3.1 and KCa2.3 inhibits in vitro and in vivo fibrotic responses [12, 13, 26, 27]. Cyclic AMP was found to reduce the expression of KCa3.1 and KCa2.3 via an Epac-mediated pathway [12], and inhibited the KCa3.1 current via PKA-dependent phosphorylation of KCa3.1 [14]. MF may exert anti-fibrotic and anti-fibrotic effects via cAMP/Epac and PKA/KCa2.3 and KCa3.1-mediated pathways in various organs, such as the liver and lungs [12].
A2AAR and A2BAR activate cAMP signaling via the Gs protein, whereas A1AR and A3AR inhibit cAMP signaling via the Gi protein. Thus, the downregulation of A2AAR and A2BAR and upregulation of A1AR and A3AR, as observed in murine models of fibrotic diseases, indicate that cAMP signaling is suppressed. Suppression of cAMP signaling may contribute to the development of fibrotic diseases. In contrast, upregulation of A2AAR and A2BAR and downregulation of A1AR and A3AR, as observed in MF-treated murine models of fibrotic diseases, indicate that cAMP signaling is augmented by MF treatment in fibrotic models. Therefore, augmented cAMP signaling may attenuate inflammatory and fibrotic progression in fibrotic diseases. These results suggest that A2AAR and A2BAR play important roles in the inhibition of liver and pulmonary fibrosis.
A2AAR is distributed in the liver, lungs, and immune system and is expressed in immune cells and fibroblasts [17]. The A2AAR acts as an endogenous modulator of inflammation and tissue repair. Expression amounts of A2AAR were markedly decreased in lung tissue from a murine model of BLM-induced pulmonary fibrosis (Fig. 1), which is consistent with the finding that A2AAR was downregulated in patients with severe IPF [28]. In addition, A2AAR-null mice were more susceptible to BLM-induced lung injuries [18]. These results suggest that A2AAR is a potential target for inflammatory and fibrotic diseases.
The role of A2B AR in the inflammatory response remains controversial. A2BAR is distributed in the bowels and lungs and expressed on immune cells [17]; in addition, A2BAR has both pro-inflammatory and anti-inflammatory effects. Pharmacological inhibition or knockout of A2BAR suppresses intestinal inflammation in murine colitis models [29] and exacerbates inflammation of dextran sodium sulfate colitis in mice [30]. Anti-inflammatory effects are generated by coupling with Gs proteins [20, 31]. Gs proteins directly interact with ACs to stimulate their catalytic activity, thereby stimulating cAMP production. As a second messenger, cAMP inhibits the production of pro-inflammatory cytokines and stimulates the production of anti-inflammatory factors through activation of the protein kinase A and Epac pathways in immune cells. On the other hand, pro-inflammatory effects are generated by coupling with Gq proteins [20, 31]. Gq proteins activate phospholipase C, and stimulated the production of pro-inflammatory cytokines such as interleukin 6 [32, 33]. However, A2BAR may be a potential target for treating acute lung injuries [34]. Pharmacological inhibition or deletion of the A2BAR enhanced pulmonary inflammation, and an A2BAR agonist attenuated pulmonary inflammation.
MF elevated cAMP levels in fibroblasts and smooth muscle cells [14], and cAMP signaling by MF was blocked by pharmacological inhibition of A2AAR and A2BAR [16]. In addition, Epac elevation by MF (Suppl. Figure 3) and anti-fibrotic effects of MF (Fig. 5a) were attenuated by inhibition of A2AAR and A2BAR. These results suggest that MF exerted anti-fibrotic effects via activation of cAMP signaling through A2AAR and A2BAR. However, it is not yet unknown whether A2A AR and A2B AR activation by MF occurs via direct or indirect mechanisms.
It is unclear how MF restores downregulated A2AAR and A2BAR in murine models of fibrotic diseases and TGFβ-treated NHLFs, and how MF blocks TGFβ-induced alterations in KCa2.3, KCa3.1, Col1, and α-SMA. It is known that MF increases cAMP levels in various cells, and cAMP regulates the gene expression of various molecules. As shown by the cAMP regulation of KCa2.3 and KCa3.1 expression, MF may regulate the expression amounts of these proteins by enhancing cAMP signaling. Cyclic AMP-induced KCa2.3 and KCa3.1 downregulation may inhibit Col1 and α-SMA production by attenuating Ca2+ signaling. However, further studies are necessary to clarify the mechanism by which MF regulates AR expression.
Chronic persistent inflammation and epithelial mesenchymal transition (EMT) are two important pathogenic events of fibrotic diseases, including IPF [35]. Therefore, the inhibition of inflammation or EMT may be an efficient therapeutic strategy for fibrotic diseases. As observed in the present study, downregulation of A2AAR and A2BAR contributed to the development of fibrotic diseases in the liver and lungs, whereas upregulation of A2AAR and A2BAR inhibited inflammatory and fibrotic responses. The activation of inflammatory and fibrotic processes, as evidenced by reduced Epac signaling and α-SMA upregulation, was inhibited by the upregulation of A2AAR and A2BAR. These results strongly suggest that A2AAR- and A2BAR-upregulating agents, such as MF, may serve as novel therapies to inhibit inflammatory and fibrotic progression via cAMP-mediated pathways.
Electronic supplementary material
List of abbreviations
- α-SMA
α-smooth muscle actin
- AC
Adenylyl cyclase
- AR
Adenosine receptor
- Bleomycin
BLM
- cAMP
Cyclic adenosine monophosphate
- CDAHFD
Choline-deficient, L-amino-acid-defined, high-fat diet with 0.1% methionine
- Col
Collagen
- DMSO
Dimethyl sulfoxide
- Epac
Exchange protein directly activated by the cAMP
- HSC
Hepatic stellate cell
- H&E
Hematoxylin and eosin
- IL
Interleukin
- IPF
Idiopathic pulmonary fibrosis
- MF
Modafinil
- MF-S
(S)-Isomer of modafinil
- MMP9
Matrix metalloproteinase-9
- MRS
MRS 1754
- NECA
5’-N-ethylcarboxamidoadenosine
- PCR
Polymerase chain reaction
- P1NP
Procollagen type1 N-terminal propeptide
- A2
AR-siRs siRNA against A2AAR + siRNA against A2BAR
- TAA
Thioacetamide
- TIMP
Tissue inhibitor of metalloproteinases
- TGFβ
Transforming growth factor-β
- TNFα
Tumor necrosis factor-α
- ZM
ZM 241,385
Haiyan Li
graduated with a B. S. in school of medicine, Eastern Liaoning University and M.D. (Hons) in department of physiology and pathophysiology, School of basic medicine, Yanbian University. She is currently studying for a PhD at Ewha Womans University in Seoul, focusing on the pathophysiology of fibrosis and the role of adenosine receptors in inflammatory and fibrotic processes. She is passionate about improving the therapeutic options that are available for treating fibrotic disorders in liver and lungs, such as nonalcoholic steatorrhea liver diseases and idiopathic pulmonary fibrosis and her aim is to continue research in this area once she has completed her PhD.
Authors’ contributions
Haiyan Li, Jee Aee Kim, Seong-Eun Jo, Huisu Lee: performed the experiments; Haiyan Li and Suk Hyo Suh: wrote the manuscipt; Kwan-Chang Kim, Shinkyu Choi, and Suk Hyo Suh: conceived and designed the research, supervised project, edited manuscript, obtained funding.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1A2C1007823), NRF grant funded by the Korea government (MSIT) (NRF-2022R1A2C1092484), Ewha Womans University Research Grant of 2022 (1-2022-1108-001-1) and intramural research promotion grants from Ewha Womans University, School of Medicine (RP-2020).
Data availability
The datasets and materials generated and analysed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethical approval
Experiments involving mice were approved by the Institutional Review Board for Human Research and Animal Care and Use Committee of the Ewha Womans University, Seoul, South Korea (EUM20-052). Experiments were conducted in accordance with the Declaration of Helsinki, Animal Care Guidelines of the Ewha Womans University Medical School, and Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85 − 23, revised in 1996).
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
KC Kim, S Choi, and SH Suh contributed equally to this publication and share therefore the corresponding authorship.
Contributor Information
Kwan-Chang Kim, Email: mdkkchang@ewha.ac.kr.
Shinkyu Choi, Email: skchoi@ewha.ac.kr.
Suk Hyo Suh, Email: shsuh@ewha.ac.kr.
References
- 1.Henderson NC, Rieder F, Wynn TA (2020) Fibrosis: from mechanisms to medicines. Nature 587(7835):555–566. 10.1038/s41586-020-2938-9 10.1038/s41586-020-2938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murtha LA, Schuliga MJ, Mabotuwana NS, Hardy SA, Waters DW, Burgess JK, Knight DA, Boyle A (2017) The processes and mechanisms of Cardiac and Pulmonary Fibrosis. Front Physiol 8:777. 10.3389/fphys.2017.00777 10.3389/fphys.2017.00777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ (2014) Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6(231):231ra47. 10.1126/scitranslmed.3008182 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield JS, Lupher M, Thannickal VJ, Wynn TA (2013) Host responses in tissue repair and fibrosis. Annu Rev Pathol 8:241–276. 10.1146/annurev-pathol-020712-163930 10.1146/annurev-pathol-020712-163930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei Q, Liu Z, Zuo H, Yang Z, Qu J (2021) Idiopathic Pulmonary Fibrosis: an update on Pathogenesis. Front Pharmacol 12:797292. 10.3389/fphar.2021.797292 10.3389/fphar.2021.797292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, Patel H, Kreuter M (2021) Global incidence and prevalence of Idiopathic Pulmonary Fibrosis. Respir Res 22(1):197. 10.1186/s12931-021-01791-z 10.1186/s12931-021-01791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholankeril G, Perumpail RB, Pham EA, Ahmed A, Harrison SA (2016) Nonalcoholic fatty Liver Disease: Epidemiology, Natural History, and Diagnostic challenges. Hepatology 64(3):954. 10.1002/hep.28719 10.1002/hep.28719 [DOI] [PubMed] [Google Scholar]
- 8.Raker VK, Becker C, Steinbrink K (2016) The cAMP pathway as therapeutic target in Autoimmune and Inflammatory Diseases. Front Immunol 7:123. 10.3389/fimmu.2016.00123 10.3389/fimmu.2016.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D, Aroonsakool N (2021) cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol 166(2):447–456. 10.1111/j.1476-5381.2012.01847.x 10.1111/j.1476-5381.2012.01847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S (2012) Protein kinase a mediated anti-inflammatory effects exerted by adenosine treatment in mouse chondrocytes stimulated with IL-1beta. BioFactors 38(6):429–439. 10.1002/biof.1040 10.1002/biof.1040 [DOI] [PubMed] [Google Scholar]
- 11.Hewer RC, Sala-Neby GB, Wu YJ, Newby AC, Bond M (2011) PKA and Epac synergistically inhibit smooth muscle cell proliferation. J Mol Cell Cardiol 50(1):87–98. 10.1016/j.yjmcc.2010.10.010 10.1016/j.yjmcc.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S, Kim JA, Li H, Jo SE, Lee H, Kim TH, Kim M, Kim SJ, Suh SH (2021) Anti-inflammatory and anti-fibrotic effects of modafinil in nonalcoholic Liver Disease. Biomed Pharmacother 144:112372. 10.1016/j.biopha.2021.112372 10.1016/j.biopha.2021.112372 [DOI] [PubMed] [Google Scholar]
- 13.Roach KM, Bradding P (2020) Ca(2+) signalling in fibroblasts and the therapeutic potential of K(ca)3.1 channel blockers in fibrotic Diseases. Br J Pharmacol 177(5):1003–1024. 10.1111/bph.14939 10.1111/bph.14939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S, Kim MY, Joo KY, Park S, Kim JA, Jung JC, Oh S, Suh SH (2012) Modafinil inhibits K(ca)3.1 currents and muscle contraction via a cAMP-dependent mechanism. Pharmacol Res 66(1):51–59. 10.1016/j.phrs.2012.02.009 10.1016/j.phrs.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 15.Brandao WN, Andersen ML, Palermo-Neto J, Peron JP, Zager A (2019) Therapeutic treatment with Modafinil decreases the severity of experimental autoimmune encephalomyelitis in mice. Int Immunopharmacol 75:105809. 10.1016/j.intimp.2019.105809 10.1016/j.intimp.2019.105809 [DOI] [PubMed] [Google Scholar]
- 16.Pal China S, Pal S, Chattopadhyay S, Porwal K, Mittal M, Sanyal S, Chattopadhyay N (2018) The wakefulness promoting drug Modafinil causes adenosine receptor-mediated upregulation of receptor activator of nuclear factor kappaB ligand in osteoblasts: negative impact of the drug on peak bone accrual in rats. Toxicol Appl Pharmacol 348:22–31. 10.1016/j.taap.2018.04.006 10.1016/j.taap.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 17.Effendi WI, Nagano T, Kobayashi K, Nishimura Y (2020) Focusing on Adenosine Receptors as a potential targeted therapy in Human Diseases. Cells 9(3):785. 10.3390/cells9030785 10.3390/cells9030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheibner KA, Boodoo S, Collins S, Black KE, Chan-Li Y, Zarek P, Powell JD, Horton MR (2009) The adenosine a2a receptor inhibits matrix-induced inflammation in a novel fashion. Am J Respir Cell Mol Biol 40(3):251–259. 10.1165/rcmb.2008-0168OC 10.1165/rcmb.2008-0168OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonioli L, Csoka B, Fornai M, Colucci R, Kokai E, Blandizzi C, Blandizzi C, Hasko G (2014) Adenosine and inflammation: what’s new on the horizon? Drug Discov Today 19(8):1051–1068. 10.1016/j.drudis.2014.02.010 10.1016/j.drudis.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Linden J (2006) New insights into the regulation of inflammation by adenosine. J Clin Invest 116(7):1835–1837. 10.1172/JCI29125 10.1172/JCI29125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vecchio EA, White PJ, May LT (2017) Targeting Adenosine receptors for the treatment of Cardiac Fibrosis. Front Pharmacol 8:243. 10.3389/fphar.2017.00243 10.3389/fphar.2017.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prisinzano T, Podobinski J, Tidgewell K, Luo M, Swenson D (2004) Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron-Asymmetry 15(6):1053–1058. 10.1016/j.tetasy.2004.01.039 10.1016/j.tetasy.2004.01.039 [DOI] [Google Scholar]
- 23.Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, Ito T, Katsume A, Sudoh M (2013) An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol 94(2):93–103. 10.1111/iep.12008 10.1111/iep.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salinthone S, Yadav V, Schillace RV, Bourdette DN, Carr DW (2010) Lipoic acid attenuates inflammation via cAMP and protein kinase A signaling. PLoS ONE 5(9):e13058. 10.1371/journal.pone.0013058 10.1371/journal.pone.0013058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S, Kim JA, Li HY, Lee SJ, Seok YS, Kim TH, Han KH, Park MH, Cho GJ, Suh SH (2019) Altered Redox State modulates endothelial K(ca)2.3 and K(ca)3.1 levels in normal pregnancy and Preeclampsia. Antioxid Redox Signal 30(4):505–519. 10.1089/ars.2017.7038 10.1089/ars.2017.7038 [DOI] [PubMed] [Google Scholar]
- 26.Organ L, Bacci B, Koumoundouros E, Kimpton WG, Samuel CS, Nowell CJ, Bradding P, Roach KM, Westall G, Jaffar J, Snibson KJ (2017) Inhibition of the K(ca)3.1 Channel alleviates established pulmonary fibrosis in a large animal model. Am J Respir Cell Mol Biol 56(4):539–550. 10.1165/rcmb.2016-0092OC 10.1165/rcmb.2016-0092OC [DOI] [PubMed] [Google Scholar]
- 27.Lee WR, Kim kh, An HJ, Kim JY, Lee SJ, Han SM, Pak KK (2014) Apamin inhibits hepatic fibrosis through suppression of transforming growth factor beta1-induced hepatocyte epithelial-mesenchymal transition. Biochem Biophys Res Commun 450(1):195–201. 10.1016/j.bbrc.2014.05.089 10.1016/j.bbrc.2014.05.089 [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR (2010) Alterations in adenosine metabolism and signaling in patients with Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. PLoS ONE 5(2):e9224. 10.1371/journal.pone.0009224 10.1371/journal.pone.0009224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasko G, Csoka B, Nemeth AH, Vizi ES, Pacher P (2009) A(2B) adenosine receptors in immunity and inflammation. Trends Immunol 30(6):263–270. 10.1016/j.it.2009.04.001 10.1016/j.it.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick JS, MacManus CF, Scully M, Glover LF, Eltzsching HK, Clogan SP (2009) Contribution of adenosine A2B receptors to inflammatory parameters of experimental Colitis. J Immunol 182(8):4957–4964. 10.4049/jimmunol.0801324 10.4049/jimmunol.0801324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune Diseases. Nat Rev Drug Discov 7(9):759–770. 10.1038/nrd2638 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng W, Song Y, Chen C, Lu ZZ, Zhang YY (2010) Stimulation of adenosine A(2B) receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-delta-P38 signalling pathway. Br J Pharmacol 159(8):1598–1607. 10.1111/j.1476-5381.2009.00558.x 10.1111/j.1476-5381.2009.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MV, Yang XL, Downey JM (2010) A(2b) adenosine receptors can change their spots. Br J Pharmacol 159(8):1595–1597. 10.1111/j.1476-5381.2010.00668.x 10.1111/j.1476-5381.2010.00668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckle T, Grenz A, Laucher S, Eltzsching HK (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118(10):3301–3315. 10.1172/JCI34203 10.1172/JCI34203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coward WR, Saini G, Jenkins G (2010) The pathogenesis of Idiopathic Pulmonary Fibrosis. Ther Adv Respir Dis 4(6):367–388. 10.1177/1753465810379801 10.1177/1753465810379801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and materials generated and analysed during the current study are available from the corresponding authors on reasonable request.