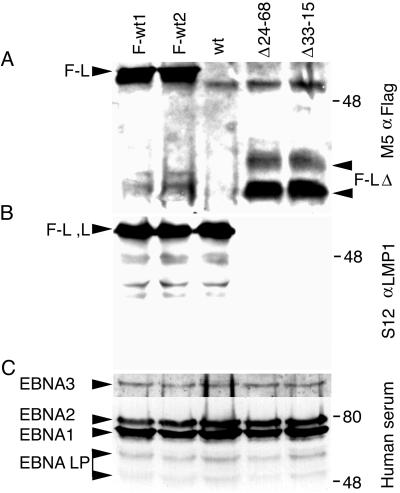
FIG. 4.
Protein expression in recombinant EBV-infected LCLs. (A) Western immunoblot analysis for Flag-LMP1. About 108 cells were Dounce homogenized in buffer containing 0.5% Brij 58, 100 mM NaCl, and 50 mM Tris (pH 7.2) and cleared by centrifugation. Flag-LMP1 was immunoprecipitated with M2 affinity gel (Sigma) for 6 h. Affinity gel was washed once, and precipitated proteins were detached with buffer containing SDS and 2-mercaptoethanol. About 10% of the immunoprecipitates were size separated in denaturing polyacrylamide gels, blotted to nitrocellulose filters, probed with M5 monoclonal antibody to Flag (Sigma) and peroxidase-conjugated secondary antibody to mouse immunoglobulin G (Amersham), and visualized by enhanced chemiluminescence (NEN Life Science). The position of Flag-LMP1 (F-L) is marked on the left, and the position of Flag-LMP1Δ232–351 (F-LΔ) is marked on the right. (B) Western immunoblot analysis for LMP1 carboxyl-terminal amino acids. About 5 × 104 cells were lysed in buffer containing SDS and 2-mercaptoethanol and resolved in denaturing polyacrylamide gels. After Western transfer to nitrocellulose filters, LMP1 was detected as in panel A, except S12 monoclonal antibody was used. The position of Flag-LMP1 (F-L) or LMP1 (L) is marked on the left. (C) Western immunoblot analysis for EBV nuclear antigens (EBNA) leader protein (LP), EBNA1, EBNA2, and EBNA3C. The position of each protein is marked on the left. Analysis was done as in panel B, except that serum from a normal human donor and peroxidase-conjugated secondary antibody to human immunoglobulin G were used. In all panels, molecular mass markers (in kilodaltons) are marked on the right.