Abstract
Cyanobacteria are the only prokaryotes capable of performing oxygenic photosynthesis on Earth. Besides their traditional roles serving as primary producers, cyanobacteria also synthesize abundant secondary metabolites including carotenoids, alkaloids, peptides, which have been reported to possess medicinal potentials. More importantly, the advancement of synthetic biology technology has further expanded their potential biomedical applications especially using living/engineered cyanobacteria, providing promising and attractive strategies for future disease treatments. To improve the understanding and to facilitate future applications, this review aims to discuss the current status and future prospects of cyanobacterial-based biomedical engineering. Firstly, specific properties of cyanobacteria related with biomedical applications like their natural products of bioactive compounds and heavy metal adsorption were concluded. Subsequently, based on these properties of cyanobacteria, we discussed the progress of their applications in various disease models like hypoxia microenvironment alleviation, wound healing, drug delivery, and so on. Finally, the future prospects including further exploration of cyanobacteria secondary metabolites, the integration of bioactive compounds synthesized by cyanobacteria in situ with medical diagnosis and treatment, and the optimization of in vivo application were critically presented. The review will promote the studies related with cyanobacteria-based biomedical engineering and its practical application in clinical trials in the future.
Keywords: Cyanobacteria, Bioactive compounds, Pathologies, Biomedical applications, Clinical application
Graphical abstract
1. Introduction
The discovery of secondary metabolites, such as antibiotics in microorganisms, has been instrumental in saving countless lives. On one hand, this advancement enables the direct use of microorganisms that produce beneficial secondary metabolites in disease diagnosis and treatment. On the other hand, these discoveries deepen our understanding of microorganisms, thereby facilitating the synthesis of these secondary metabolites in non-host organisms through cutting-edge technologies like synthetic biology. For instance, Paddon and Keasling et al. achieved a groundbreaking milestone by ingeniously engineering the fermentation process of Saccharomyces cerevisiae to produce artemisinic acid [1]. This was followed by extraction and chemical conversion to produce the antimalarial drug artemisinin. The semi-synthetic method offers an alternative route for artemisinin production, supplementing the existing plant-based approach and contributing to the global supply of artemisinin. Additionally, Davar et al. reported the first human clinical trial of live bacteria fecal microbiota transplantation (FMT) with living bacteria for anti-PD-1 immunotherapy in patients with metastatic melanoma [2], which showed evidences of clinical benefit in a subset of treated patients. An increasing number of studies have demonstrated the promising future for the biomedical application of microorganisms.
Cyanobacteria, commonly known as blue-green algae, are the sole prokaryotes capable of performing oxygenic photosynthesis on Earth [3]. They are among the earliest organisms to inhabit the Earth, appearing as early as 3.3 to 3.5 billion years ago [4]. Cyanobacteria are significant producers of oxygen and fixers of nitrogen, playing a crucial role in shaping ecosystems and the biosphere [5]. They have been extensively studied by researchers as model microorganisms, particularly in the areas of photosynthesis [[6], [7], [8]], CO2 fixation [[9], [10], [11]], and circadian rhythm [[12], [13], [14]]. Traditionally, cyanobacteria are also abundant in secondary metabolites, including carotenoids [15], alkaloids [16], peptides [17], which have been reported to possess medicinal potential. Among them, Arthrospira (Spirulina) is one of the most successful cases of commercialization due to their abundant accumulation of protein, vitamins and minerals [18,19], which has also been employed in various clinical treatments [[20], [21], [22]]. The advancement of synthetic biology technology has further expanded the potential biomedical applications of cyanobacteria [23], including treatment of tumor models [24], heavy metals [25], wound healing [26], drug delivery [26], etc. The studies demonstrated significant potentials in utilizing cyanobacteria (both biologically active compounds and living cells) for disease treatment or as adjuvant therapy. In addition, the advancement of novel technologies like 3D bioprinting and microfluidics has significantly advanced the utilization of microorganisms, including cyanobacteria, in biomedicine. One representative example is the 3D printing of photosynthetic microorganisms as living materials, commonly employed for oxygen delivery and wound healing [27,28]. These living materials exhibit high biocompatibility and photosynthetic activity, offering a promising solution to address hypoxia in biomedical applications.
To improve the understanding and to facilitate future applications, this review aims to discuss the status and future prospects of cyanobacterial-based biomedical engineering especially using living and/or engineered cyanobacteria constructed via synthetic biology, which is an important complementation and update to related reviews published in recent years [23,29,30]. Specifically, the properties of cyanobacteria relevant to biomedical applications, such as their production of bioactive compounds and ability to adsorb heavy metals, were reviewed with a particular focus on the latest developments. Subsequently, based on these properties of cyanobacteria, we discussed the advancements in their applications, particularly those driven by synthetic biology and emerging technologies such as 3D bioprinting and microfluidics. These applications include various disease models, such as alleviating hypoxic microenvironments, wound healing, and drug delivery. Finally, the prospects were critically presented. Further exploration of cyanobacteria secondary metabolites (such as cyanotoxin), the integration of bioactive compounds synthesized by cyanobacteria in situ with medical diagnosis and treatment, and the optimization of in vivo application, will better promote the practical application of cyanobacteria in clinical trials.
2. Potential biomedical properties of cyanobacteria
2.1. Natural products of cyanobacteria
Numerous studies have examined a wide range of natural products from cyanobacteria, primarily encompassing the following aspects (Fig. 1): i) bioactive compounds such as terpenoids, pigment proteins, alkaloids, and lipopeptides synthesized within the cell; ii) oxygen production; iii) exopolysaccharides (EPS), a water-soluble polysaccharide secreted into the extracellular space; iv) extracellular vesicles (EVs) secreted outside the cell. Currently, over 2000 cyanobacterial secondary metabolites have been identified from various sources. These data have been compiled in several databases, including Antibase, MarinLit, Cyanomet, and CyanoMetDB [31]. Many of these compounds like scytoscalarol, phycocyanin, and tjipanazoles have been evaluated for their antibacterial, anti-inflammatory, and antiviral properties [32]. Cyanobacteria, the sole prokaryotic microalgae capable of producing oxygen, exhibit remarkable rates of photosynthetic oxygen production. Their efficiency surpasses that of land plants by a factor of 10 and exceeds that of algae by a factor of two [10]. Leveraging the inherent oxygen-generating capacity of cyanobacteria, they can be served as oxygen donors, providing a sustainable source of reactive oxygen for oxygen-depleted diseases [33]. Cyanobacteria have evolved the capability to synthesize and release EPS into the cell periphery and surrounding environment as an adaptive mechanism to cope with complex and variable environments. The anionic nature of most cyanobacterial EPS is attributed to the presence of charged groups, such as uronic acid and sulfate groups, which are also responsible for the antiviral properties of polysaccharides. Consequently, EPS derived from cyanobacteria holds promise for applications in pharmaceuticals and other industries [34]. Bacterial-synthesized compounds can be released from the cell membrane in the form of vesicles to form EVs. While many bacterial-derived EVs have been employed for biomedical applications, the applications of cyanobacterial EVs have been less documented [35]. Nevertheless, given the appealing characteristics of cyanobacterial EVs, promising applications can still be anticipated. This section will highlight recent research advancements related to the synthesis of aforementioned natural products.
Fig. 1.
Potential biomedical properties of cyanobacteria including natural products, heavy metals absorption, and CO2 conversion.
2.1.1. Bioactive compounds
Cyanobacteria have the capability to synthesize a wide array of bioactive compounds in response to various environmental stresses, enabling their widespread geographical distribution. Key bioactive substances found in cyanobacteria encompass terpenoids, pigment proteins, alkaloids, and lipopeptides, etc. (representative compounds were listed in Table 1). These compounds contribute to the diverse biochemical potential of cyanobacteria, presenting numerous opportunities for application across various fields, particularly in biotechnology and biomedicine [36].
Table 1.
Biomedical properties of bioactive compounds from cyanobacteria. (N/A, not available).
| Compounds | Species | Properties | Test strategy | Doses | Ref. |
|---|---|---|---|---|---|
| Terpenoids | |||||
| Astaxanthin | N/A | Anti-inflammatory | Incubation | 25 μM | [37] |
| Carotenoids | Nodosilinea antarctica | Anti-inflammatory | Incubation | 100 μg dry extract/mL | [38] |
| Carotenoids |
Pseudanabaena Spirulina Lyngbya |
Anti-inflammatory | N/A | 40 mg dry extract/mL | [39] |
| Scytoscalarol | Scytonema | Anti-bacteria | Microdilution method | 40 μg crude extract/mL | [40] |
| Cybastacines | Nostoc | Anti-fungal | Kirby-Bauer agar disk diffusion method | ≤2 μg/mL | [41] |
| Scytonemides | Scytonema hofmannii | Anti-cancer | Injection | >20 μg/mL | [42] |
| Pigment proteins | |||||
| Phycocyanin | Limnothrix | Anti-cancer | Incubation, | 250 μg/mL, | [43] |
| Phycocyanin | Anabaena oryzae | Anti-bacteria | Agar well-diffusion assay, hydrogels | 100 μg/mL, 100 μL |
[44,45] |
| Alkaloids | |||||
| Tjipanazoles | Calothrix | Anti-cancer | Incubation | 58 nM | [46] |
| Welwitindolinone | N/A | Anti-cancer | N/A | 0.1 μM | [47,48] |
| Lipopeptides | |||||
| Pseudo-dysidenine and dragonamide | Lyngbya majuscula | Anti-cancer | N/A | >1 μg/mL | [49] |
| Somocystinamide A | L. majuscula | Anti-cancer | Incubation, N/A, |
≥3 nM, 100 nM, |
[50,51] |
| EPS | Crypthecodinium cohnii | Anti-inflammatory | Incubation, | 800 μg/mL | [52] |
|
Chlorella pyrenoidosa Scenedesmus Chlorococcum |
Anti-cancer | Incubation | 0.6 mg/mL | [53] | |
| Gyrodinium impudicum | Anti-virus | MTT assay | 26.9 μg/mL | [54] | |
| Cochlodinium polykrikoides, | Anti-virus | MTT assay | 100 μg/mL | [55] | |
| Aliinostoc | Wound healing | Incubation | 10 μg/mL | [56] | |
| Nostoc | Wound healing | Hydrogels | 1%w/v | [57] | |
Terpenoids, also known as isoprenoids, form the largest class of natural products with a broad range of commercial uses, including pharmaceuticals, food additives, and industrial chemicals. Among the most extensively studied terpenoids in cyanobacteria are squalene, amorphadiene, farnesene, isoprene, beta-phellandrene, geranyllinalool, limonene, bisabolene and astaxanthin [36,58]. Among them, Kazunori et al. reported the effectiveness of squalene in reducing neuroinflammation and modulating neurotransmitter systems [59]. Additionally, Callie et al. illustrated that astaxanthin possesses anti-inflammatory and antioxidant properties, making it a potential agent for inflammation prevention [37].
Pigments derived from cyanobacteria and microalgae, such as chlorophyll, carotenoids, and phycobilin, exhibit valuable properties for industrial and biomedical applications [60]. The terpenoids mentioned above include β-carotene and astaxanthin, which are both carotenoids. Phycobilin, also known as phycobiliproteins (PBPs), are produced by various algae, including Cryptomonads, Glaucophytes, Rhodophyta, and particularly cyanobacteria. PBPs consist of phycocyanin, allophycocyanin, and phycoerythrin, with phycocyanin being the most prevalent and widespread [15]. The cyanobacterium Arthrospira platensis is the primary source for phycocyanin production, and extensive research has been conducted in this area [[61], [62], [63], [64]]. Furthermore, the synthesis of phycocyanin in other algae has also been documented, such as Synechocystis [65], Synechococcus [66], Phormidium [67], Plectonema [68], and Pyropia yezoensis [69].
Alkaloids, a class of nitrogen-containing organic compounds, are commonly found in nature, primarily in plants, animals, and cyanobacteria. They typically exhibit complex ring structures, with nitrogen predominantly present within the ring. Alkaloids are significant active components in Chinese herbal medicine, with the isolation of key alkaloids like morphine and quinine driving significant progress of medicine in the 19th century. Consequently, alkaloids will continue to serve as valuable biological tools in the development of new drugs [16]. Cyanobacteria are known to naturally produce various alkaloids [16,70], including anatoxin, saxitoxin, β-N-methylamino-l-alanine, cylindrospermopsin, and indole alkaloids. These cyanobacterial alkaloid metabolites possess distinct chemical structures and exhibit diverse biological activities, offering promising avenues for physiological and pharmacological research.
Lipopeptide compounds exhibit significant diversity and hold substantial potential for applications in antibiotics, pharmaceuticals, and biocontrol agents. Xue et al. reviewed 18 cyclic lipopeptides and 13 linear lipopeptides sourced from cyanobacteria, as well as various cyanobacteria-derived compounds [71]. Notably, pseudo-dysidenine and dragonamide, both derived from Lyngbya majuscula, demonstrated potent inhibitory effects on cancer cells [49]. Additionally, somocystinamide A and Kalkitoxin, also isolated from the same species, exhibited strong anticancer properties as well [50,72].
2.1.2. Photosynthetic oxygen production capacity
Oxygenic photosynthesis is a crucial metabolic process that has significantly influenced the evolution of life and ecology on Earth. This process has led to the accumulation of oxygen in the atmosphere, which has had a profound impact on the planet's history [73]. The Great Oxygenation Event (GOE), occurring approximately 2.4 billion years ago, marked a significant increase in atmospheric oxygen levels [74]. Cyanobacteria, as one of the primary agents of oxygenic photosynthesis, play a pivotal role in this process. Both geological evidence [75] and genomic studies [76] indicate that the ancestors of cyanobacteria likely initiated oxygenic photosynthesis approximately 500 million years before the GOE. The increasing application of cyanobacteria in biotechnology is closely linked to its photosynthetic efficiency. Cyanobacteria utilize only a fraction of sunlight due to the limited light wavelength of absorption range (approximately 400–700 nm) of the pigments in their cells [77]. While the theoretical maximum photosynthetic energy conversion efficiency of cyanobacteria and microalgae can reach 4.5%–9%, this efficiency will decrease to less than 3% in bioreactors [78], leaving significant room for improvement. Numerous studies have focused on enhancing the photosynthetic capacity of cyanobacteria, including efforts to broaden the light absorption spectrum [79] and mitigate energy loss caused by photoinhibition or oxidative stress [80,81]. In recent years, the advancement and utilization of diverse biomaterials have led to the widespread application of cyanobacteria and other algae in addressing hypoxia diseases, including tumor [82] and wound healing [83] et al., owing to their inherent photosynthetic oxygen production capabilities. Of interest, Maharjan et al. demonstrated the 3D fabrication of a perfused vascularized tissue construct using Chlamydomonas reinhardtii [84], which served as sustainable biomimetic oxygen concentrators to enhance the viability and function of human hepatocytes in vitro. Microalgae, particularly cyanobacteria, are primary producers responsible for generating 50%–80% of the Earth's oxygen [85]. Consequently, extensive research has been conducted on cyanobacterial oxygen evolution. For instance, Liang et al. overexpressed carbon flux control enzymes in Synechocystis sp. PCC 6803 [86], resulting in a 115% increase in intracellular oxygen evolution. Similarly, Porcellinis et al. achieved enhanced photosynthetic capacity and oxygen evolution in Synechococcus sp. PCC 7002 by overexpressing a bifunctional diphosphatase [87]. Furthermore, Kędzior et al. developed a method for quantitatively determining the photosynthetic oxygen evolution rate of S. elongatus PCC 7942 using oxygen electrodes [88], providing a valuable framework for studying cyanobacterial oxygen evolution.
2.1.3. Exopolysaccharides (EPS)
Since the 1960s, over 200 species of cyanobacteria have been identified as capable of secreting extracellular polysaccharides [34]. Notably, polysaccharides from classes like Nostoc, Arthrospira and Microcoleus have been the focus of numerous studies. The intricate and varied structures of EPS, coupled with their easy accessibility in the extracellular milieu, have garnered interest across various fields and industries. For instance, EPS find extensive applications in numerous chemical products such as gums, bioflocculants, biosorbents, and soil conditioners [89].
The earliest investigations into EPS production by microalgae trace back to 1964, where a monosaccharide component was discovered in the mucosal polymer of Palmella mucosa [90]. Subsequent research over the decades has highlighted significant EPS accumulation in various species including Anabaena, Calothrix marchica, Cyanospira capsulate, Cyanothece, Leptolyngbya, Plectonema, and Nostoc [91]. Additionally, Synechococcus [92], Oscillatoria [92], Synechocystis [93], and Cyanobium [93] have been identified as promising candidates for EPS production. As a matter of fact, the application of bacterial-derived EPS in biomedical research has been more extensively explored compared to microalgae-derived EPS [94]. For instance, a recent study on marine bacteria Alteromonas sp. PRIM-28 revealed that the EPS produced by this strain were biocompatible [95]. The EPS effectively stimulated the proliferation and migration of epidermal fibroblasts and keratinocytes, indicating the potential of this EPS as a bioactive agent in wound healing applications. In addition, Niknezhad et al. synthesized an ultra-elastic hydrogel utilizing EPS extracted from Pantoea sp. BCCS 001. This hydrogel was then employed in the treatment of volumetric muscle loss in rats [96]. Similarly, numerous cyanobacteria and microalgae have also demonstrated the ability to produce EPS, with reported anti-inflammatory and antioxidant properties. For instance, EPS derived from Crypthecodinium cohnii have been shown to inhibit inflammation by modulating toll-like receptor 4 (TLR4) [52]. Furthermore, Zhang et al. reported that Chlorella pyrenoidosa, Chlorococcum, and Scenedesmus produce EPS with antioxidant properties [53]. Additionally, some cyanobacteria-derived EPS have been used to develop insecticide encapsulated nanocarriers to enhance the effect of insecticides due to their bioactive functions and favorable material characteristics [97]. Consequently, EPS have emerged as promising candidates for biomedical uses owing to their distinct biological activities.
2.1.4. Extracellular vesicles (EVs)
EVs are spherical structures ranging from approximately 20 to 400 nm in diameter, consisting of vesicles enclosed by a phospholipid bilayer and lacking the ability to self-replicate. EVs contain a variety of biomolecules, including soluble proteins, lipids, membrane proteins, nucleic acids, and other metabolites, acting as carriers for intercellular transport [98]. Initially discovered in the outer membrane, EVs are commonly referred to as outer membrane vesicles (OMVs). However, recent studies have unveiled other vesicle formation processes. Based on their formation pathways, vesicles are further classified as outer-inner-membrane vesicles (OIMVs), explosive outer-membrane vesicles (EOMVs), and tube-shaped membranous structures (TSMSs) [99]. Regardless of their synthesis pathway, all these vesicles fall under the category of EVs.
Numerous reports have documented EVs produced by cyanobacteria. For instance, Biller et al. [100] detected and compared the proteomes associated with EVs produced by two distinct Prochlorococcus strains (MED4 and MIT9313). These vesicles were found to contain a diverse array of proteins, including nutrient transporters, proteases, porins, hydrolases, and many proteins of unknown function. Furthermore, Pardo et al. [101] identified OMVs in the freshwater algae Synechocystis sp. PCC 6803, excluding the presence of cytoplasmic and thylakoid membrane components, thus confirming the outer membrane origin of these vesicles. This represents the first related report in this biotechnologically important species. Additionally, EVs have been detected in Synechococcus sp. PCC 7002 [102], Synechococcus sp. WH8102 [100], S. elongatus PCC 7942 [103], Cyanothece sp. CCY 0110 [104], Jaaginema litorale LEGE 07176 [105], Anabaena sp. PCC 7120 [106], Cylindrospermopsis raciborskii CYRF-01 [107], and Azolla microphylla [108]. EVs exhibit the capacity to package and transport cargo to other targets. Their external features can be engineered and loaded with cargo through bioengineering and synthetic biology techniques. Consequently, EV-based nanotechnology has rapidly advanced and found applications in various biotechnology fields, holding promise for significant contributions to biological clinical research [35].
2.2. Heavy metal adsorption and accumulation capacity
Heavy metals pose a global threat to humans and the environment, with cadmium (Cd), mercury (Hg), lead (Pb), and arsenic (As) being the most common toxic heavy metals that endanger human health and the ecological environment. Even at low concentrations, they exhibit highly toxic, carcinogenic, and teratogenic properties [109]. Conventional heavy metal treatment methods are costly and inefficient, and traditional metal ion chelating agents are not suitable for addressing subchronic and chronic heavy metal toxicity [110,111]. Cyanobacteria have been utilized in soil and water pollution control due to their photosynthetic autotrophic ability to thrive in various extreme environments [112]. Additionally, cyanobacteria exhibit high affinity and multiple binding sites for heavy metals, making them valuable in heavy metal bioremediation efforts [113,114].
Cyanobacteria adsorb heavy metal ions through covalent bond formation, ion exchange between ionized cell walls and heavy metal ions, or by binding heavy metal cations to negatively charged exopolysaccharides (EPS) [115,116]. Cyanobacteria commonly utilize the second binding mode for heavy metal adsorption [117]. Numerous studies have demonstrated that different functional groups of EPS, such as amide, carbonyl, carboxylic acid, ether, and hydroxyl group, enhance its adsorption capacity [118,119]. This underscores the cyanobacteria's ability to effectively adsorb heavy metals. Heavy metals can infiltrate cyanobacterial cells, and certain substances within the cytoplasm can bind these metals, preventing them from reaching toxic levels. Metal-binding proteins (MBPs) represent a significant group of cytoplasmic metal-binding entities in cyanobacteria, with metallothioneins (MTs) being the largest subgroup, capable of binding heavy metals through the sulfhydryl group of their cysteine [120]. Cyanobacterial cells also harbor polyphosphate bodies that can serve to enrich heavy metals within the cells [121]. Furthermore, phytochelatins (PCs), well-known metal-binding peptides in higher plants, eukaryotic algae, and fungi, can also bind heavy metals intracellular [122].
2.3. CO2 conversion capacity
Cyanobacteria have the capacity to harness light energy and CO2 for photosynthetic autotrophic growth, leading to the production of various beneficial chemicals through their metabolic processes. An increasingly prominent approach involves the utilization of genetically modified cyanobacteria to convert CO2 into biofuels and other valuable chemical products through photosynthesis [123].
Several cyanobacteria strains have been investigated for their potential in chemical production [123,124]. Many of these strains are easily genetically manipulable, and as interest in engineering cyanobacteria grows, the available metabolic engineering tools for such strains are expanding [125]. The production of chemicals has also been largely confirmed in cyanobacteria. Here, we mainly discuss some examples of cyanobacteria producing medicinal functional chemicals. For instance, Hilzinger et al. [126] demonstrated the synthesis of acetaminophen in A. platensis, a common drug ingredient for analgesia and antipyresis. Additionally, astaxanthin, a natural carotenoid with potent antioxidant, anti-tumor, anti-inflammatory, and immune stimulatory properties, has been successfully produced by H. pluvialis, reaching up to 4 % by dry cell weight (DCW) and is widely used for industrial astaxanthin production [127]. Furthermore, Diao et al. [128] achieved the production of 29.6 mg/gDCW of astaxanthin in Synechocystis sp. PCC 6803. Another example is the production of myo-inositol, widely used in functional food and pharmaceutical industries due to its potential role in treating Alzheimer's disease and hyperglycemia. Wang et al. [129] achieved the production of 12.72 mg/L myo-inositol also in the cyanobacterium Synechocystis sp. PCC 6803. Furthermore, using S. elongatus UTEX 2973 as a chassis, Sun et al. achieved real-time regulation using multiple artificial small RNAs and myo-inositol sensors, ultimately achieving a myo-inositol production of 262.6 mg/L [130]. Moreover, certain cyanobacteria strains have demonstrated the ability to produce significant amounts of sugars, particularly sucrose, through synthetic biology approaches. Santos-Merino et al. have provided a comprehensive review on this topic [131]. Sucrose, a fermentable disaccharide, can be utilized as a carbon source by many heterotrophic bacteria. Consequently, it's expected to develop an artificial co-culture systems comprising sucrose-secreting cyanobacteria and heterotrophic microorganisms to realize the one-step conversion of cyanobacterial-secreted sucrose to high-value products. It also provides a new reference for designing artificial microbial alliances for medical applications in the future.
3. Advances in biomedical applications of cyanobacteria
3.1. Biomedical applications of cyanobacterial natural products
Extensive research on cyanobacteria has increasingly unveiled the unique structure and multifunctional biological activities of their natural products [132]. These compounds have garnered significant interest across various fields, particularly in medicine [133]. They exhibit important medical properties, including antibacterial, antifungal, anticancer, immunosuppressive, anti-inflammatory, and antioxidant effects [134,135]. For instance, cyanobacteria are rich sources of omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which offer protection against cardiovascular disease [136]. Table 2 summarizes the biomedical applications of cyanobacteria and their bioactive compounds. Numerous pertinent reviews are available and will not be reiterated here [24,137,138].
Table 2.
The utilization of cyanobacteria and their bioactive compounds in the field of biomedicine. (N/A, not available).
| Species | Properties | Model | Diseases | Test strategy | Doses | Ref. |
|---|---|---|---|---|---|---|
| Aurantiochytrium | Anti-inflammatory | Mice | Neuroinflammation | Oral | 100 mg/kg | [59] |
| Arthrospira platensis, Dunaliella Salina | Anti-cancer | Hamster | Tumors | Injection | 350 μg/0.3 mL | [139] |
| Spirulina platensis | N/A | Rats | Liver injury | Liver perfusion | 0.1 and 0.2 mg/ml/g of liver | [140] |
| S. platensis | Anti-cancer | Rats | Colon cancer | Oral | 200 mg/kg | [141] |
| S. platensis | Anti-inflammatory | Mice | Colitis | Oral | 150 mg/kg | [142] |
| Lyngbya majuscula | Anti-cancer | Mice | Breast cancer | Incubation, Injection |
80 nM-3 μM, 10 mg/kg |
[50,72] |
| Porphyridium | Anti-virus | Rats and rabbits | Herpes simplex viruses | Injection/scratching | 100 μg/mL | [143] |
| Phormidium | Anti-inflammatory | Zebrafish | N/A | Incubation | 6.25–100 μg/mL | [144] |
| Synechocystis | Toxicity | Zebrafish | N/A | Immersion trials | 250 and 500 μg LPS/mL of EVs | [145] |
| Synechococcus elongatus | Wound healing | Mice | N/A | Injection | 105 cells/100 μL | [103] |
| Chlorella vulgaris | Oxygen therapy | Mice | Cancer | Injection, Injection, Injection |
106 cells/mL, 8 × 106 cells/mL, 107 cells/mL |
[33] [146,147] |
| S. elongatus | Oxygen therapy | Mice | Myocardial injury | Injection | 106 cells | [148] |
| S. elongatus | Oxygen therapy | Mice | Rheumatoid arthritis | Injection | 108 cells | [149] |
| S. elongatus | Oxygen therapy | Rats | Anaerobic infectious keratitis and infected periodontitis | Hydrogel | 4 × 107 cells/mL | [150] |
|
C. reinhardtii Synechocystis |
Oxygen therapy | Xenopus laevis tadpoles | Neuronal hypoxia | Injection | 1010 cells/mL | [151] |
| C. pyrenoidosa | Oxygen therapy/wound healing | Mice | Diabetic wounds | Hydrogel | 105-107 cells/mL | [152] |
| C. vulgaris | Heavy metal biosorption | Mice | Lead poisoning | Gavage | 8 mg/mL | [153] |
| S. elongatus | Provide carbon source | Rats | Skin defect | Hydrogel | 1.5 × 108 cells/mL | [154] |
| S. platensis | Drug delivery | Mice | Diabetic wounds | Hydrogel | 800 μg/mL | [155] |
| S. platensis | Drug delivery | Mice | Cancer | Injection | 500 μg (DCW) | [156] |
| S. platensis | Drug delivery | Mice | Radiation-induced injury | Oral | 0.5 mg/mL | [157] |
3.1.1. Biomedical applications of bioactive compounds
Carotenoids and their derivatives from five cyanobacteria have high levels of superoxide anion radical (O2•−) scavenging activity and potential applications in psoriasis treatment. Lopes et al. [38] evaluated their anti-inflammatory effects on LPS-induced macrophages (RAW 264.7), with an IC50 of 0.3 mg/mL. In another case, Paliwal et al. [39] extracted carotenoids from Pseudanabaena, Arthrospira, and Lyngbya and tested them in vitro as effective agents against urinary calculi. Moreover, Schwartz and Shklar [139] suggested using a carotenoid-rich extract (from A. platensis and D. salina) as an antineoplastic agent against 7,12-dimethylbenzanthracene (DMBA)-induced hamster tumors, resulting in local regression of cancer cells within 4–8 weeks. Furthermore, other terpenoids such as scytoscalarol, cybastacines and scytonemides, exhibited antibacterial, antifungal and anticancer activity [[40], [41], [42]].
Phycocyanin, like carotenoids, exhibits potent antioxidant effects [158]. Gdara et al. [140] proposed that phycocyanin mitigates liver injury by reducing the activity of oxidative stress-activating enzymes, such as alkaline phosphatase and liver transaminase. In a study by Gantar et al. [43], a type of prostate cancer cell was treated with a combination of the anticancer drug topotecan and phycocyanin (derived from Limnothrix). Similarly, Saini et al. [141] observed a 70 % increase in efficacy when combining piroxicam and phycocyanin compared to using the single drug for treating colon cancer in mice. Additionally, phycocyanin demonstrated significant anti-inflammatory effects. Daily supplementation of selenium-enriched phycocyanin (150 mg/kg) from A.platensis effectively suppressed colitis, bloody diarrhea, and weight loss in mice [142]. In addition to the aforementioned functions, phycocyanin also plays a role in antibacterial activity and promoting wound healing [44,45]. Indoles alkaloids represent the most commonly employed alkaloids in pharmaceutical research. Tjipanazoles exhibit an IC50 of approximately 100 nM against Plasmodium falciparum, and this category of indole alkaloids also hinders the proliferation of HeLa cancer cells with an IC50 of 40 nM [46]. The semi-inhibitory concentration of Welwitindolinone in breast and ovarian cancer cells also reaches the μM level [47,48]. In addition to the anticancer properties described above, lipopeptide compounds derived from cyanobacteria mainly exhibit significant therapeutic potential in the treatment of parasitic and bacterial infections [71,159].
3.1.2. Cyanobacteria based bio-oxygen pump
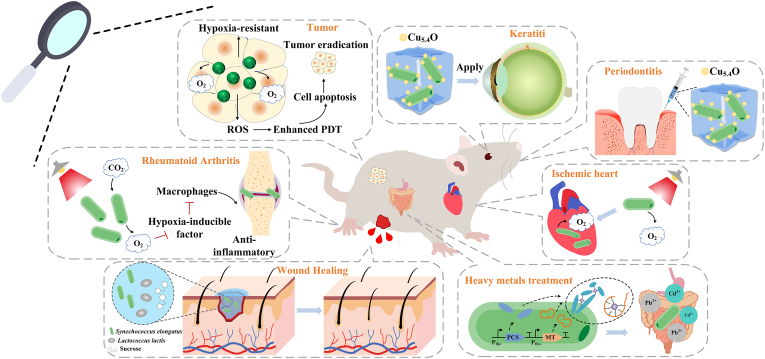
Organ damage and organ failure are significant medical concerns. A crucial issue in human tissue injury, affecting organs such as the brain, heart, skin, muscle, and pancreas, is the continuous supply of oxygen and nutrients to prevent severe hypoxia during culture, a process known as oxygenation [26]. Cyanobacteria exhibit high photosynthetic efficiency and can continuously convert H2O into oxygen under light conditions. This ability makes them suitable for providing local oxygen supply. Consequently, they can serve as sources for highly oxygen-dependent treatments such as reactive oxygen therapy and photodynamic therapy, thereby improving therapeutic outcomes (see Fig. 2) [33].
Fig. 2.
Applications of cyanobacteria-based biomedical engineering via animal models.
Tumors often face local hypoxia, presenting a significant challenge in cancer treatment, particularly for radiotherapy (RT) and photodynamic therapy (PDT) [160]. Addressing hypoxia-based resistance to conventional cancer therapies requires effective reoxygenation of tumors [161]. Qiao et al. [33] introduced a method to generate O2 in situ within mice tumors through red light-induced photosynthesis. They employed red cell membrane (RBCM) to encapsulate C. vulgaris, delivering RBCM-microalgae to the tumor, resulting in O2 generation under red light irradiation, thereby enhancing tissue oxygenation and improving RT. Li et al. [146] and Zhong et al. [147] have also proposed a microalgae-based RT/PDT combination therapy, similar to Qiao et al. [33], but with surface modification using medically common non-immunogenic and environmentally friendly biomaterials such as silica and calcium phosphate. In a study by Zhou et al. [162], the application of Chlorella and calcium alginate as oxygen-supplying agents in PDT treatment of 4T1 tumor model in mice resulted in significant tumor regression around the treated area. The use of algae implanted near the tumor site enhanced the effectiveness of the PDT treatment, leading to notable improvements in tumor response. Additionally, Sun et al. [163] utilized S. elongatus as a bio-oxygen pump and incorporated indocyanine green (ICG) as a photosensitizer loaded on mesoporous silica nanoparticles (MSN) within hydrogels. These hydrogels were then administered via injection into breast tumor mice, resulting in notable therapeutic effects on the tumors. This innovative cancer treatment approach holds promise for replacing current treatment methods in the future.
The use of oxygen-releasing therapy represents a recent advancement in ischemic diseases. Cohen et al. [148] reported the successful application of S. elongatus to mitigate myocardial injury following ischemia. S. elongatus, in the absence of blood flow, can absorb CO2 produced by ischemic cardiomyocytes in vivo and produce O2 through photosynthesis to support metabolic activity. Building on this research, Stapleton et al. [164] enhanced the therapeutic efficacy of cyanobacteria by encapsulating them in alginate microgels.
Furthermore, cyanobacteria have also shown promise in addressing other hypoxia diseases. For instance, Guo et al. [149] developed a cyanobacteria micro-nano device to continuously generate oxygen in rheumatoid arthritis (RA) joints, leading to the down-regulation of hypoxia-inducible factor-1α (HIF-1α). This approach reduces the number of M1 macrophages and induces the polarization of M2 macrophages, offering potential for treating RA. The hypoxic environment in refractory infections, such as refractory keratitis and periodontitis, poses challenges for the application of PDT. In a study by Wang et al. [150], S. elongatus PCC 7942 was delivered into anaerobic infectious keratitis and infected periodontitis rat models using the photosensitizer (Ce6) and ultra-small Cu5.4O nanoparticles (Cu5.4O USNPs) with peroxidase activity as a carrier. The results demonstrated a significant alleviation of the restriction of PDT by the hypoxic environment, with a positive effect on inflammation. Additionally, the ability of cyanobacteria to act as bio-oxygen pumps in vivo was highlighted in another study by Özugur et al. [151]. In this study, C. reinhardtii and Synechocystis sp. PCC6803 were injected into the brains of hypoxic Xenopus laevis tadpoles. The study showed that when neuronal activity ceased completely due to hypoxia, the oxygen produced by photosynthetic microorganisms reliably triggered neuronal restart and rescue. As an additional compelling example, Wang et al. proposed a novel living photosynthetic scaffold designed to adapt to irregularly shaped wounds and enhance their healing through the utilization of an in situ microfluidic-assisted 3D bioprinting strategy [152]. By directly applying a complete layer of live microalgae support onto diabetic wounds, they can effectively alleviate local hypoxia, stimulate angiogenesis, and promote extracellular matrix (ECM) synthesis, thereby significantly accelerating the closure of chronic wounds.
3.1.3. Biomedical applications of cyanobacterial EPS
Cyanobacterial EPS have garnered increasing attention due to their renewability, rheological properties, high yield, and their potential applications in various biotechnological fields, particularly in biomedicine. They have been recognized for their pharmacological roles as antioxidants, antiviral, antibacterial, antitumoral, and immunomodulatory agents [34]. For instance, EPS derived from Porphyridium marinum exhibited minimal inhibitory concentrations (MIC) of 0.125 g/L against Staphylococcus aureus, and demonstrated effectiveness against Escherichia coli, Salmonella enteritidis, and Methicillin-Resistant S. aureus. Several EPS isolates from cyanobacteria and microalgae have also shown antiviral activity against respiratory viruses, influenza virus, HIV-1 virus, herpes virus, and encephalomyocarditis virus [54,55,143]. Moreover, Zhang et al. [53] reported the use of EPS produced by C. pyrenoidosa, Scenedesmus, and Chlorococcum in treating human colon cancer cell lines HCT116 and HCT8, demonstrating anti-tumour activity. Zampieri et al. [144] extracted EPS components from Phormidium and tested their anti-inflammatory and pro-catabolic activities on zebrafish inflammation models, revealing positive effects on inflammation and non-toxicity to zebrafish. Additionally, EPS from Nostoc, and Alinostoc have shown bioactivity in promoting wound healing [56,57]. Despite the various pharmacological properties of cyanobacterial EPS, many therapeutic mechanisms remain to be fully elucidated. Nonetheless, this does not diminish the potential of cyanobacterial EPS as a highly promising drug for biomedical applications.
3.1.4. Biomedical applications of cyanobacterial EVs
In recent years, EVs have been increasingly investigated as strong candidates for biomedical applications, especially for certain gram-negative bacteria [165,166]. However, most reports on cyanobacterial EVs focus on the physiological characteristics of EVs. In the future, tools for the application of cyanobacterial EVs may be developed based on research into the application of other types of bacteria. Matinha-Cardoso et al. [145] conducted a study in which they co-cultured Synechocystis EVs with zebrafish fry. The results indicated that the Synechocystis EVs were biocompatible with the fish and did not induce toxicity. Additionally, reporter proteins were employed to evaluate the delivery of the EVs in zebrafish. This study offers initial evidence supporting the potential of cyanobacterial EVs as carriers for drug delivery, thus establishing a basis for future related research. Yin et al. [103] demonstrated the potential of cyanobacterial EVs for biomedical applications by using EVs secreted by S. elongatus PCC 7942 to promote endothelial cell angiogenesis interleukin-6 (IL-6), resulting in wound repair and regeneration in mice skin.
It is worth noting that cyanobacterial LPS is significantly less toxic than LPS from other Gram-negative bacteria and also possesses potential anti-inflammatory and other medical properties [167,168]. Additionally, cyanobacteria naturally synthesize compounds with medicinal effects. These substances can be incorporated into EVs through genetic engineering, which may become a research focus in the biomedical field for cyanobacterial EVs in the future.
3.2. Cyanobacteria-based heavy metal treatments
Biosorption, which harnesses the natural adsorption capacity of microorganisms to remove heavy metals from the environment, presents a promising remediation method for heavy metal contamination. While various microorganisms such as cyanobacteria (microalgae), bacteria, fungi, and yeasts exhibit biosorption capabilities, not all are suitable for in vivo heavy metal detoxification studies, as some may be pathogenic [169]. Notably, microalgae, including cyanobacteria, demonstrate exceptional heavy metal ion adsorption capacity, along with greater biosafety and broader healthcare applications compared to other microorganisms [33,170,171]. However, research related to the detoxification of heavy metals by microalgae remains limited to mechanistic studies and environmental remediation, with only a few studies exploring medical and healthcare applications. For instance, Liu et al. [153] developed an oral, cross-linked microalgal hydrogel system comprising C. vulgaris (CV) and berberine (BBR) that degrades lead. In their study, the microalgae hydrogel degraded in the alkaline environment of the mice gut, gradually releasing BBR and CV. CV facilitated biosorption and maintained a healthy intestinal microenvironment, while BBR bolstered antioxidant defenses for tissues and organs. The biological gel effectively removed lead ions, inhibited lead-induced inflammation in the liver, kidney, and colon, reduced tissue damage, and demonstrated biodegradability and high biosafety, thus holding potential for clinical applications. Recently, Sun et al. genetically engineered the cyanobacterium Synechocystis sp. PCC 6803 by introducing genes encoding phytochelatins (PCSs) and metallothioneins (MTs) [172]. To facilitate the application of Synechocystis, a sodium alginate-based hydrogel embedding technique was employed in conjunction with 3D printing. The modified bacteria demonstrated remarkable efficacy in rescuing zebrafish from Cd2+ contaminated water in vitro, as well as significantly reducing Cd2+ content in mice and restoring their normal activity behavior. We anticipate that these examples will serve as a starting point for the medical application of cyanobacterial biosorption of heavy metals. Furthermore, we expect that there will be further exploration in this field in the future, driven by the advantages of cyanobacterial biosorption.
3.3. Cyanobacteria-based microbial consortia therapy
Microbial consortia have existed for a long time, such as natural flora in soil or intestinal flora in animals, but they have gained significant attention in recent years [173]. Combinations or associations of microorganisms often result in metabolic capacities that are different and more robust than those of a single strain. Various studies have explored engineered microbial communities that maintain structural stability through strategies such as mutualism and symbiosis [174,175]. Cyanobacteria, through photosynthesis, can convert CO2 to other hydrocarbons, particularly sugars, which can serve as a carbon source for other heterotrophic microorganisms. Additionally, the cultivation cost of cyanobacteria is very low, making it highly suitable for co-culture with other microorganisms. Cyanobacteria-based microbial consortia have been designed with bacteria, yeast, and other microalgae [[176], [177], [178], [179], [180]].
While existing studies are primarily focused on metabolic engineering, it is these foundational and physiological activity studies that can offer valuable references for the use of cyanobacteria-based microbial consortia for treatment. Notably, Li et al. [154] recently designed and fabricated a photoautotrophic "living" bandage containing engineered microbial flora. The microbial consortia consisted of S. elongatus PCC 7942, which produces sucrose, and heterotrophic engineered bacteria (E. coli or Lactococcus lactis) that utilize sucrose as a carbon source and synthesize functional biomolecules. Subsequently, its role in promoting wound healing was demonstrated in a full-thickness rat skin defect model. This study serves as an excellent example of the application of cyanobacterial-based microbial consortia in disease treatment.
3.4. Drug delivery
Current drug delivery systems often suffer from several common drawbacks, including poor drug solubility, short effective duration of action, and low bioavailability [181]. In recent years, cyanobacteria (microalgae) have emerged as one of the most promising drug delivery systems due to their good biocompatibility, low cost, large surface area with active surface, phototropism, and high propulsion [182,183].
Shchelik et al. [184] developed a novel drug delivery system by covalently linking vancomycin to the surface of C. reinhardtii, using azadibenzocylooctyne-amine (DBCO) as a chemical adhesive. This system can release vancomycin under light conditions and synergize with ultraviolet therapy to treat skin infections. Hu et al. [155] loaded berberine (BBR), an antibacterial agent, into S. platensis and combined it with carboxymethyl chitosan/sodium alginate to form a bioactive hydrogel. Under laser irradiation, the composite gel can continuously release BBR and produce reactive oxygen species. This study verified that the composite gel can promote angiogenesis, skin regeneration, and inhibit inflammation, thus aiding in the healing of methicillin-resistant S. aureus (MRSA)-infected diabetic wounds. Zhong et al. [156] also utilized Arthrospira as a carrier to achieve targeted delivery of doxorubicin. Drug loading was achieved through noncovalent electrostatic interactions between positively charged doxorubicin and negatively charged Arthrospira surfaces. This approach demonstrated excellent targeted therapy ability in the 4T1 breast cancer lung metastasis mice model. Recently, Zhang et al. [157] employed the same algae combined with astaxanthin nanoparticles to construct an oral microalga-nanocomposite system, which showed promising anti-inflammatory effects. The unique biological structure and movement ability of microalgae, such as cyanobacteria, provide new ideas for the design of precise targeted drug delivery systems. Their active surface can efficiently load small molecule drugs, avoiding the complex steps of chemical synthesis and expensive raw materials, making algae have broad application prospects in the field of drug delivery.
4. Future prospective and direction
4.1. Development of novel secondary metabolites
In addition to the natural products mentioned earlier, cyanobacteria can produce various secondary metabolites, primarily cyanotoxins, some of which have demonstrated pharmacological effects (see Fig. 3) [185]. Over the past few decades, an increasing number of studies have focused on cyanotoxins, including identifying their chemical structures, elucidating biosynthetic pathways, exploring toxic effects, and investigating biotechnology applications [31]. Recent studies have suggested that cyanotoxins may also be potential candidates in drug development due to their biological activity. For instance, saxitoxin can be used as an anesthetic in combination with other drugs to enhance the anesthetic effect by blocking nerve channels [186]. Additionally, apratoxin and cryptophycin have demonstrated the ability to combat cancer cells [187,188]. However, to fully utilize the medicinal properties of cyanobacteria secondary metabolites, more attention should be given to exploring cyanobacteria as a source of medical compounds, screening novel antibacterial compounds, and fully understanding the mechanism of action of cyanobacteria secondary metabolites. Further studies are still needed to achieve these goals.
Fig. 3.
Future prospects of cyanobacteria-based biomedical engineering.
4.2. Medical testing of engineered cyanobacteria
Cyanobacteria, as photosynthetic autotrophic prokaryotes, have been a significant focus of research in synthetic biology due to their potential for various biological applications, such as environmental remediation and chemical production [189]. However, to date, the use of engineered cyanobacteria in medical research, particularly in the treatment of heavy metal ions, has been limited, with most studies concentrating solely on their environmental applications. Furthermore, there is limited documentation on the in-situ synthesis of bioactive compounds by engineered cyanobacteria for diagnosis and treatment. The current practice primarily involves the utilization of wild-type cyanobacteria as carriers for drug delivery or as suppliers of oxygen in specific hypoxia conditions. At most, engineered cyanobacteria are utilized to provide a carbon source for other heterotrophic microorganisms capable of producing bioactive substances. The potential of cyanobacteria for medical applications remains largely unexplored, and there has been minimal research in this area [3]. In the future, it is crucial to integrate engineered cyanobacteria with medical testing to explore their potential in medical research. This integration will promote to unlock new opportunities for the use of cyanobacteria in medicine.
4.3. Optimization of in vivo applications
Cyanobacteria rely on continuous exposure to light for their cultivation and various physiological and biochemical activities, particularly oxygen production. However, in the process of diagnosis and treatment, especially in vivo applications, it is challenging for light to penetrate through the skin and other tissues into the body. Developing novel lighting technologies that address issues such as light penetration through different tissues presents further challenges. Among them, red light and near-red light (NIR) have been utilized for cyanobacteria-based diagnosis and treatment due to their superior ability to penetrate the skin compared to other wavelengths of light. For instance, Zhang et al. [190,191] applied red light in cyanobacterial hydrogel tumor therapy and a probiotic system that modulates the gut-brain axis respectively. Wang et al. [192], on the other hand, developed NIR-driven oxygenic cyanobacteria for ischemic stroke treatment while Liu et al. [193] employed NIR-driven cyanobacterial nanocapsules for prevention and treatment of myocardial infarction. Nevertheless, although these studies addressed concerns regarding light transmission, they were conducted using mouse models as therapeutic targets. In contrast, human skin is denser and less permeable to light penetration. Moreover, since most human tissues are not adapted to cope with optical radiation effectively yet phototoxicity remains unclear in this context. Therefore, there is still a need to develop a customized lighting strategy capable of inducing photosynthetic oxygen production in both internal and external organs of the human body.
4.4. In vivo biosafety of engineered cyanobacteria
The use of genetically engineered microorganisms for diagnosis and treatment presents significant biosafety concerns, which may entail potential risks to human health and the environment [194]. Furthermore, their genomic instability and the potential to initiate new diseases have impeded their clinical application. To address the issues of cell leakage and uncontrolled proliferation of therapeutically engineered cyanobacteria for implantation, strategies involving physical containment have been explored. For instance, hydrogels with highly hydrated polymer networks are commonly utilized for cell encapsulation [195].
Another significant biosafety concern is antibiotic resistance resulting from horizontal gene transfer. This poses a substantial risk to the genetic stability of ecosystems, a process that may be accelerated by advances in synthetic biology [196]. To tackle these issues, strategies such as physical encapsulation, active anchoring of bacteria, auxotrophy, and suicide switches can be individually or collectively applied to confine genetically engineered cyanobacteria and address safety concerns [197].
While cyanotoxins produced in cyanobacteria exhibit potent biological activities, they are highly toxic to mammals. Despite a few cyanotoxins entering clinical trials, only a few have gained approval from the FDA. Consequently, the modification or elimination of cyanotoxins is unavoidable for the further utilization of cyanobacteria in clinical therapy. Accelerating the annotation of the biosynthetic pathway and structure of cyanotoxins is imperative to determine their toxicity. Additionally, genetic engineering technology can be employed to modify or eliminate the highly toxic cyanotoxins, thereby regulating and mitigating their toxicity. This would reduce cyanobacteria toxicity, facilitating their potential application in clinical medicine.
The human body harbors diverse microbial populations, many of which play a role in regulating human metabolism and conferring benefits. However, cyanobacteria contain various antibacterial substances, such as terpenoids and peptides. When these substances enter the human body, they may cause irreversible damage to the body's probiotic community, impacting microbial diversity and disrupting human metabolism, thus posing a risk to health. Therefore, further investigation into the interaction mechanism between cyanobacteria bioactive compounds and human microorganisms is necessary. Subsequently, synthetic biology methods can be employed to adjust the effects, facilitating the potential application of cyanobacteria in vivo.
In summary, genetically engineered microorganisms must undergo biosafety assessments before practical use. Additionally, the development of standards is imperative to classify different therapeutic microorganisms, and the establishment of new legislative and regulatory constraints is crucial to evaluate the biosafety and ethical considerations of research and the clinical translation of these innovative diagnostic and therapeutic organisms.
4.5. Clinical trials
Cyanobacteria (microalgae), have been regarded as promising candidates for drug development since their discovery. However, comprehensive clinical evaluations of cyanobacteria are still necessary prior to their approval for diagnostic purposes. The unique characteristics of cyanobacteria as microorganisms complicate their preclinical and clinical analysis, necessitating more extensive investigation and posing challenges in fitting them into a rigid regulatory framework. Currently, only A. platensis, A. maxima, H. pluvialis, Schizochytrium, and Ulkenia woken have obtained food safety certifications from the US and EU authorities. Among these species, only Arthrospira has been utilized in a limited number of clinical trials [[20], [21], [22],198]. Recent studies have shown the advantages of Arthrospira in treating obesity, hypertension, and diabetes [199]. A randomized double-blind trial investigated the combined impact of Arthrospira supplementation (4.5 g/day for 6 weeks) and a structured exercise regimen in dyslipidemic individuals [20]. The study revealed synergistic advantages, with Arthrospira supplementation alone leading to reductions in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride levels, alongside an increase in high-density lipoprotein cholesterol (HDL-C) within the 6-week period compared to the control group. Moreover, a recent randomized double-blind placebo-controlled trial involved 60 type 2 diabetes patients receiving standard metformin treatment [200]. Results demonstrated that consuming 2 g of spirulina before meals significantly enhanced blood glucose parameters, including glycosylated hemoglobin (HbA1c) and fasting blood glucose levels (FBS), compared to the placebo group receiving metformin alone. These clinical investigations affirm the safety of Arthrospira consumption and showcase its beneficial effects in various diseases, thereby encouraging its potential for future clinical applications. Conversely, studies on numerous other cyanobacteria are still far from reaching the stage of clinical trials. Despite some validation in mice models, the actual application in human clinical research remains distant and significantly delays the evaluation of their potential. In the future, there is also a need to enhance our understanding and design bioactive engineered cyanobacteria to fill the developmental pipeline.
5. Conclusions
This article provides an overview of the application of cyanobacteria (microalgae) in the biomedical field. Firstly, the abundant library of secondary metabolites like bioactive compounds, EPS, EVs and O2 provided important candidates for biomedicines. Among them, EVs have been demonstrated efficient for chemical or nucleic drugs delivery. In addition, biomedical applications of cyanobacteria achieved significant progresses in cancer therapy, wound healing and heavy metals bioremediation using different models. Among them, Arthrospira, as one of the leading microalgae strains, has successfully progressed to clinical trials, with several studies showcasing its advantageous role in treating obesity and cardiovascular disease. While the literature reviewed emphasizes the potential benefits of cyanobacteria and microalgae, it is important to note that in vitro assays and animal models may not fully reflect the complexity of human diseases. Pharmacokinetic issues such as product bioavailability, efficacy, and safety also pose challenges. Another significant obstacle is the presence of neurotoxins in many active compounds derived from cyanobacteria, complicating their use in medical applications. Furthermore, factors such as race, lifestyle, behavior, and gender-specific differences can influence the physiological response to treatment and should be considered in large-scale studies to enhance efficacy and safety.
CRediT authorship contribution statement
Tong Zhang: Writing – original draft. Dailin Liu: Conceptualization. Yingying Zhang: Conceptualization. Lei Chen: Supervision. Weiwen Zhang: Writing – review & editing, Supervision. Tao Sun: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by grants from the National Key Research and Development Program of China (Grant no. 2019YFA0904600 National Natural Science Foundation of China (Grant nos. 32270091, 31972931 and 32070083).
Contributor Information
Weiwen Zhang, Email: wwzhang8@tju.edu.cn.
Tao Sun, Email: tsun@tju.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Paddon C.J., Westfall P.J., Pitera D.J., Benjamin K., Fisher K., McPhee D., Leavell M.D., Tai A., Main A., Eng D., Polichuk D.R., Teoh K.H., Reed D.W., Treynor T., Lenihan J., Fleck M., Bajad S., Dang G., Dengrove D., Diola D., Dorin G., Ellens K.W., Fickes S., Galazzo J., Gaucher S.P., Geistlinger T., Henry R., Hepp M., Horning T., Iqbal T., Jiang H., Kizer L., Lieu B., Melis D., Moss N., Regentin R., Secrest S., Tsuruta H., Vazquez R., Westblade L.F., Xu L., Yu M., Zhang Y., Zhao L., Lievense J., Covello P.S., Keasling J.D., Reiling K.K., Renninger N.S., Newman J.D. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 2.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., Zidi B., Zhang S., Badger J.H., Vetizou M., Cole A.M., Fernandes M.R., Prescott S., Costa R.G.F., Balaji A.K., Morgun A., Vujkovic-Cvijin I., Wang H., Borhani A.A., Schwartz M.B., Dubner H.M., Ernst S.J., Rose A., Najjar Y.G., Belkaid Y., Kirkwood J.M., Trinchieri G., Zarour H.M. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dismukes G.C., Carrieri D., Bennette N., Ananyev G.M., Posewitz M.C. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008;19(3):235–240. doi: 10.1016/j.copbio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Schopf J.W., Packer B.M. Early archean (3.3-billion to 3.5-billion-year-old) microfossils from warrawoona group, Australia. Science. 1987;3(237):70–73. doi: 10.1126/science.11539686. [DOI] [PubMed] [Google Scholar]
- 5.Whitton B.A., Potts M. Springer; Dordrecht: 2012. Introduction to the cyanobacteria, ecology of cyanobacteria II; pp. 1–13. [Google Scholar]
- 6.Oliver T., Kim T.D., Trinugroho J.P., Cordón-Preciado V., Wijayatilake N., Bhatia A., Rutherford A.W., Cardona T. The evolution and evolvability of photosystem II. Annu. Rev. Plant Biol. 2023;74(1):225–257. doi: 10.1146/annurev-arplant-070522-062509. [DOI] [PubMed] [Google Scholar]
- 7.Calzadilla P.I., Kirilovsky D. Revisiting cyanobacterial state transitions. Photochem. Photobiol. Sci. 2020;19(5):585–603. doi: 10.1039/c9pp00451c. [DOI] [PubMed] [Google Scholar]
- 8.Ho M.-Y., Soulier N.T., Canniffe D.P., Shen G., Bryant D.A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr. Opin. Plant Biol. 2017;37:24–33. doi: 10.1016/j.pbi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Borden J.S., Savage D.F. New discoveries expand possibilities for carboxysome engineering. Curr. Opin. Microbiol. 2021;61:58–66. doi: 10.1016/j.mib.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J., Sun H., Chen R., Sun J., Mo G., Luan G., Lu X. Multiple routes toward engineering efficient cyanobacterial photosynthetic biomanufacturing technologies. Green Carbon. 2023;1(2):210–226. doi: 10.1016/j.greenca.2023.11.004. [DOI] [Google Scholar]
- 11.Kupriyanova E.V., Pronina N.A., Los D.A. Adapting from low to high: an update to CO2-concentrating mechanisms of cyanobacteria and microalgae. Plants. 2023;12(7) doi: 10.3390/plants12071569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swan J.A., Golden S.S., LiWang A., Partch C.L. Structure, function, and mechanism of the core circadian clock in cyanobacteria. J. Biol. Chem. 2018;293(14):5026–5034. doi: 10.1074/jbc.TM117.001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKnight B.M., Kang S., Le T.H., Fang M., Carbonel G., Rodriguez E., Govindarajan S., Albocher-Kedem N., Tran A.L., Duncan N.R., Amster-Choder O., Golden S.S., Cohen S.E. Roles for the Synechococcus elongatus RNA-binding protein Rbp2 in regulating the circadian clock. J. Biol. Rhythm. 2023;38(5):447–460. doi: 10.1177/07487304231188761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Li S., Sun T., Zong Y., Luo Y., Wei Y., Zhang W., Zhao K. Oscillation of type IV pili regulated by the circadian clock in cyanobacterium Synechococcus elongatus PCC7942. Sci. Adv. 2024;10(4) doi: 10.1126/sciadv.add9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chini Zittelli G., Lauceri R., Faraloni C., Silva Benavides A.M., Torzillo G. Valuable pigments from microalgae: phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023;22(8):1733–1789. doi: 10.1007/s43630-023-00407-3. [DOI] [PubMed] [Google Scholar]
- 16.Vasas G., Borbely G., Nánási P., Nánási P.P. Alkaloids from cyanobacteria with diverse powerful bioactivities. Mini-Rev. Med. Chem. 2010;10(10):946–955. doi: 10.2174/138955710792007231. [DOI] [PubMed] [Google Scholar]
- 17.Gogineni V., Hamann M.T. Marine natural product peptides with therapeutic potential: chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta Gen. Subj. 2018;1862(1):81–196. doi: 10.1016/j.bbagen.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosshagauer S., Kraemer K., Somoza V. The true value of spirulina. J. Agric. Food Chem. 2020;68(14):4109–4115. doi: 10.1021/acs.jafc.9b08251. [DOI] [PubMed] [Google Scholar]
- 19.Lafarga T., Fernández-Sevilla J.M., González-López C., Acién-Fernández F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109356. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Lepe M.A., Wall-Medrano A., Lopez-Diaz J.A., Juarez-Oropeza M.A., Hernandez-Torres R.P., Ramos-Jimenez A. Hypolipidemic effect of Arthrospira (spirulina) maxima supplementation and a systematic physical exercise Program in overweight and obese men: a double-blind, randomized, and crossover controlled trial. Mar. Drugs. 2019;17(5) doi: 10.3390/md17050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szulinska M., Gibas-Dorna M., Miller-Kasprzak E., Suliburska J., Miczke A., Walczak-Gałezewska M., Stelmach-Mardas M., Walkowiak J., Bogdanski P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension a randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017;21(10):2473–2481. [PubMed] [Google Scholar]
- 22.Miczke A., Szulińska M., Hansdorfer-Korzon R., Kręgielska-Narożna M., Suliburska J., Walkowiak J., Bogdański P. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians a double-blind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016;20(1):150–156. [PubMed] [Google Scholar]
- 23.Huang H., Lang Y., Zhou M. A comprehensive review on medical applications of microalgae. Algal Res. 2024 doi: 10.1016/j.algal.2024.103504. [DOI] [Google Scholar]
- 24.Baig M.S., Rajpoot S., Ohishi T., Savai R., Seidel S., Kamennaya N.A., Bezsonov E.E., Orekhov A.N., Mahajan P., Solanki K., Saqib U. Anti-lung cancer properties of cyanobacterial bioactive compounds. Arch. Microbiol. 2022;204(10) doi: 10.1007/s00203-022-03194-0. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya S. The role of spirulina (Arthrospira) in the mitigation of heavy-metal toxicity: an appraisal. J. Environ. Pathol. Toxicol. Oncol. 2020;39(2):149–157. doi: 10.1615/JEnvironPatholToxicolOncol.2020034375. [DOI] [PubMed] [Google Scholar]
- 26.Dawiec-Lisniewska A., Podstawczyk D., Bastrzyk A., Czuba K., Pacyna-Iwanicka K., Okoro O.V., Shavandi A. New trends in biotechnological applications of photosynthetic microorganisms. Biotechnol. Adv. 2022;59 doi: 10.1016/j.biotechadv.2022.107988. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Xue Y., Zhang T., Fang Q., Jin M., Wang X., Wang Z., Hu Y., Zhao W., Lou D., Tan W.Q. Photosynthetic biomaterials: applications of photosynthesis in algae as oxygenerator in biomedical therapies. Bio-Design and Manufacturing. 2021;4(3):596–611. doi: 10.1007/s42242-021-00129-4. [DOI] [Google Scholar]
- 28.Obaíd M.L., Camacho J.P., Brenet M., Corrales-Orovio R., Carvajal F., Martorell X., Werner C., Simón V., Varas J., Calderón W., Guzmán C.D., Bono M.R., San Martín S., Eblen-Zajjur A., Egaña J.T. A first in human trial implanting microalgae shows safety of photosynthetic therapy for the effective treatment of full thickness skin wounds. Front. Med. 2021;8 doi: 10.3389/fmed.2021.772324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahra Z., Choo D.H., Lee H., Parveen A. Cyanobacteria: review of current potentials and applications. Environments. 2020;7(2) doi: 10.3390/environments7020013. [DOI] [Google Scholar]
- 30.Khavari F., Saidijam M., Taheri M., Nouri F. Microalgae: therapeutic potentials and applications. Mol. Biol. Rep. 2021;48(5):4757–4765. doi: 10.1007/s11033-021-06422-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z., Zhu X., Wu Z., Sun T., Tong Y. Recent advances in cyanotoxin synthesis and applications: a comprehensive review. Microorganisms. 2023;11(11) doi: 10.3390/microorganisms11112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abed R.M., Dobretsov S., Sudesh K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009;106(1):1–12. doi: 10.1111/j.1365-2672.2008.03918.x. [DOI] [PubMed] [Google Scholar]
- 33.Qiao Y., Yang F., Xie T., Du Z., Zhong D., Qi Y., Li Y., Li W., Lu Z., Rao J., Sun Y., Zhou M. Engineered algae A novel oxygen-generating system for effective treatment of hypoxic cancer. Sci. Adv. 2020;6(21) doi: 10.1126/sciadv.aba5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laroche C. Exopolysaccharides from microalgae and cyanobacteria: diversity of strains, production strategies, and applications. Mar. Drugs. 2022;20(5) doi: 10.3390/md20050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima S., Matinha-Cardoso J., Tamagnini P., Oliveira P. Extracellular vesicles: an overlooked secretion system in cyanobacteria. Life. 2020;10(8) doi: 10.3390/life10080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouyahya A., Bakrim S., Chamkhi I., Taha D., El Omari N., El Mneyiy N., El Hachlafi N., El-Shazly M., Khalid A., Abdalla A.N., Goh K.W., Ming L.C., Goh B.H., Aanniz T. Bioactive substances of cyanobacteria and microalgae: sources, metabolism, and anticancer mechanism insights. Biomed. Pharmacother. 2023;170 doi: 10.1016/j.biopha.2023.115989. [DOI] [PubMed] [Google Scholar]
- 37.Farruggia C., Kim M.B., Bae M., Lee Y., Pham T.X., Yang Y., Han M.J., Park Y.K., Lee J.Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Lopes G., Clarinha D., Vasconcelos V. Carotenoids from cyanobacteria: a biotechnological approach for the topical treatment of psoriasis. Microorganisms. 2020;8(2) doi: 10.3390/microorganisms8020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paliwal C., Ghosh T., Bhayani K., Maurya R., Mishra S. Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar. Drugs. 2015;13(8):5384–5401. doi: 10.3390/md13085384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo S., Krunic A., Pegan S.D., Franzblau S.G., Orjala J. An antimicrobial guanidine-bearing sesterterpene from the cultured cyanobacterium Scytonemasp. J. Nat. Prod. 2009;72(11):1911–2080. doi: 10.1021/np900288x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabanillas A.H., Tena Perez V., Maderuelo Corral S., Rosero Valencia D.F., Martel Quintana A., Ortega Domenech M., Rumbero Sanchez A. Cybastacines A and B: antibiotic sesterterpenes from a Nostoc sp. Cyanobacterium, J Nat Prod. 2018;81(2):410–413. doi: 10.1021/acs.jnatprod.7b00638. [DOI] [PubMed] [Google Scholar]
- 42.Krunic A., Vallat A., Mo S., Lantvit D.D., Swanson S.M., Orjala J. Scytonemides A and B, cyclic peptides with 20S proteasome inhibitory activity from the cultured cyanobacterium Scytonema hofmanii. J. Nat. Prod. 2010;73(11):1927–1932. doi: 10.1021/np100600z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gantar M., Dhandayuthapani S., Rathinavelu A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food. 2012;15(12):1091–1095. doi: 10.1089/jmf.2012.0123. [DOI] [PubMed] [Google Scholar]
- 44.Sitohy M., Osman A., Ghany A.G.A., Salama A. Antibacterial phycocyanin from Anabaena oryzae SOS13. International Journal of Applied Research in Natural Products. 2015;8(4):27–36. [Google Scholar]
- 45.Dev A., Mohanbhai S.J., Kushwaha A.C., Sood A., Sardoiwala M.N., Choudhury S.R., Karmakar S. kappa-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring. Acta Biomater. 2020;109:121–131. doi: 10.1016/j.actbio.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Rickards R.W., Rothschild J.M., Willis A.C., de Chazal N.M., Kirk J., Kirk K., Saliba K.J., Smith G.D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron. 1999;55(47):13513–13520. doi: 10.1016/S0040-4020(99)00833-9. [DOI] [Google Scholar]
- 47.Smith C.D., Zilfou J.T., Stratmann K., Patterson G.M., Moore R.E. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995;47(2):241–247. [PubMed] [Google Scholar]
- 48.Zhang X., Smith C.D. Microtubule effects of welwistatin, a cyanobacterial indolinone that circumvents multiple drug resistance. Mol. Pharmacol. 1996;49(2):288–294. [PubMed] [Google Scholar]
- 49.Jiménez J.I., Scheuer P.J. New lipopeptides from the caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001;64(2):200–203. doi: 10.1021/np000462q. [DOI] [PubMed] [Google Scholar]
- 50.Wrasidlo W., Mielgo A., Torres V.A., Barbero S., Stoletov K., Suyama T.L., Klemke R.L., Gerwick W.H., Carson D.A., Stupack D.G. The marine lipopeptide somocystinamide A triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. U.S.A. 2008;105(7):2313–2318. doi: 10.1073/pnas.0712198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan J.B., Liu Y., Coothankandaswamy V., Mahdi F., Jekabsons M.B., Gerwick W.H., Valeriote F.A., Zhou Y.D., Nagle D.G. Kalkitoxin inhibits angiogenesis, disrupts cellular hypoxic signaling, and blocks mitochondrial electron transport in tumor cells. Mar. Drugs. 2015;13(3):1552–1568. doi: 10.3390/md13031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma X., Xie B., Du J., Zhang A., Hao J., Wang S., Wang J., Cao G. The anti-inflammatory effect and structure of EPCP1-2 fromCrypthecodinium cohniivia modulation of TLR4-NF-kappaB pathways in LPS-induced RAW 264.7 cells. Mar. Drugs. 2017;15(12) doi: 10.3390/md15120376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Liu L., Ren Y., Chen F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019;128:761–767. doi: 10.1016/j.ijbiomac.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Yim J.H., Kim S.J., Ahn S.H., Lee C.K., Rhie K.T., Lee H.K. Antiviral effects of sulfated exopolysaccharide from the marine microalga gyrodinium impudicum strain KG03. Mar. Biotechnol. 2004;6(1):17–25. doi: 10.1007/s10126-003-0002-z. [DOI] [PubMed] [Google Scholar]
- 55.Masahiko Hasui M.M., Okutani Koichi, Shigeta Shiro. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995;17(5):293–297. doi: 10.1016/0141-8130(95)98157-t. [DOI] [PubMed] [Google Scholar]
- 56.Demay J., Halary S., Knittel-Obrecht A., Villa P., Duval C., Hamlaoui S., Roussel T., Yepremian C., Reinhardt A., Bernard C., Marie B. Anti-inflammatory, antioxidant, and wound-healing properties of cyanobacteria from thermal mud of balaruc-les-bains, France: a multi-approach study. Biomolecules. 2020;11(1) doi: 10.3390/biom11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alvarez X., Alves A., Ribeiro M.P., Lazzari M., Coutinho P., Otero A. Biochemical characterization of Nostocsp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021;254 doi: 10.1016/j.carbpol.2020.117303. [DOI] [PubMed] [Google Scholar]
- 58.Lin P.C., Pakrasi H.B. Engineering cyanobacteria for production of terpenoids. Planta. 2019;249(1):145–154. doi: 10.1007/s00425-018-3047-y. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki K., Othman M.B., Ferdousi F., Yoshida M., Watanabe M., Tominaga K., Isoda H. Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deepika C., Wolf J., Roles J., Ross I., Hankamer B. Sustainable production of pigments from cyanobacteria. Adv. Biochem. Eng. Biotechnol. 2023;183:171–251. doi: 10.1007/10_2022_211. [DOI] [PubMed] [Google Scholar]
- 61.Huo Y., Hou X., Yu Y., Wen X., Ding Y., Li Y., Wang Z. Improving the thermal and oxidative stability of food-grade phycocyanin from Arthrospira platensis by addition of saccharides and sugar alcohols. Foods. 2022;11(12) doi: 10.3390/foods11121752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan M., Zhao H., Guo J., Yan L., Zhang D., Bai W., Li Y. Comparison of C-phycocyanin from extremophilic Galdieria sulphuraria and Spirulina platensis on stability and antioxidant capacity. Algal Res. 2021;58 doi: 10.1016/j.algal.2021.102391. [DOI] [Google Scholar]
- 63.Bocker L., Hostettler T., Diener M., Eder S., Demuth T., Adamcik J., Reineke K., Leeb E., Nystrom L., Mathys A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020;316 doi: 10.1016/j.foodchem.2020.126374. [DOI] [PubMed] [Google Scholar]
- 64.Patil G., Chethana S., Madhusudhan M.C., Raghavarao K.S. Fractionation and purification of the phycobiliproteins fromSpirulina platensis. Bioresour. Technol. 2008;99(15):7393–7396. doi: 10.1016/j.biortech.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 65.Puzorjov A., Mert Unal S., Wear M.A., McCormick A.J. Pilot scale production, extraction and purification of a thermostable phycocyanin from Synechocystissp. PCC 6803. Bioresour. Technol. 2022;345 doi: 10.1016/j.biortech.2021.126459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antecka A., Klepacz-Smółka A., Szeląg R., Pietrzyk D., Ledakowicz S. Comparison of three methods for thermostable C-phycocyanin separation and purification. Chemical Engineering and Processing - Process Intensification. 2022;171 doi: 10.1016/j.cep.2021.108563. [DOI] [Google Scholar]
- 67.Avci S., Haznedaroglu B.Z. Pretreatment of algal and cyanobacterial biomass for high quality phycocyanin extraction. J. Appl. Phycol. 2022;34(4):2015–2026. doi: 10.1007/s10811-022-02770-7. [DOI] [Google Scholar]
- 68.Roy D., Pabbi S. A simple improved protocol for purification of C-phycocyanin from overproducing cyanobacteria and its characterization. J. Appl. Phycol. 2022;34(2):799–810. doi: 10.1007/s10811-021-02649-z. [DOI] [Google Scholar]
- 69.Zang F., Qin S., Ma C., Li W., Lin J. Preparation of high-purity R-phycoerythrin and R-phycocyanin fromPyropia yezoensis in membrane chromatography. J. Appl. Phycol. 2020;32(5):3411–3418. doi: 10.1007/s10811-020-02208-y. [DOI] [Google Scholar]
- 70.Hohlman R.M., Sherman D.H. Recent advances in hapalindole-type cyanobacterial alkaloids: biosynthesis, synthesis, and biological activity. Nat. Prod. Rep. 2021;38(9):1567–1588. doi: 10.1039/d1np00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xue Y., Zhao P., Quan C., Zhao Z., Gao W., Li J., Zu X., Fu D., Feng S., Bai X., Zuo Y., Li P. Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides. 2018;107:17–24. doi: 10.1016/j.peptides.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Shrestha S.K., Min K.H., Kim S.W., Kim H., Gerwick W.H., Soh Y. Kalkitoxin: a potent suppressor of distant breast cancer metastasis. Int. J. Mol. Sci. 2023;24(2) doi: 10.3390/ijms24021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez-Baracaldo P., Cardona T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020;225(4):1440–1446. doi: 10.1111/nph.16249. [DOI] [PubMed] [Google Scholar]
- 74.Bekker A., Holland H.D., Wang P.L., Rumble D., J H., Hannah J.L., Coetzee L.L., Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427(6970):117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 75.Planavsky N.J., Asael D., Hofmann A., Reinhard C.T., Lalonde S.V., Knudsen A., Wang X., Ossa Ossa F., Pecoits E., Smith A.J.B., Beukes N.J., Bekker A., Johnson T.M., Konhauser K.O., Lyons T.W., Rouxel O.J. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 2014;7(4):283–286. doi: 10.1038/ngeo2122. [DOI] [Google Scholar]
- 76.Cardona T., Murray J.W., Rutherford A.W. Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria. Mol. Biol. Evol. 2015;32(5):1310–1328. doi: 10.1093/molbev/msv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hitchcock A., Hunter C.N., Sobotka R., Komenda J., Dann M., Leister D. Redesigning the photosynthetic light reactions to enhance photosynthesis - the PhotoRedesign consortium. Plant J. 2022;109(1):23–34. doi: 10.1111/tpj.15552. [DOI] [PubMed] [Google Scholar]
- 78.Wijffels R.H., Barbosa M.J. An outlook on microalgal biofuels. Science. 2010;329(5993):796–799. doi: 10.1126/science.118900. [DOI] [PubMed] [Google Scholar]
- 79.Wolf B.M., Blankenship R.E. Far-red light acclimation in diverse oxygenic photosynthetic organisms. Photosynth. Res. 2019;142(3):349–359. doi: 10.1007/s11120-019-00653-6. [DOI] [PubMed] [Google Scholar]
- 80.Lea-Smith D.J., Ross N., Zori M., Bendall D.S., Dennis J.S., Scott S.A., Smith A.G., Howe C.J. Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystissp. PCC 6803 to survive rapidly changing light intensities. Plant Physiol. 2013;162(1):484–495. doi: 10.1104/pp.112.210260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hakkila K., Antal T., Rehman A.U., Kurkela J., Wada H., Vass I., Tyystjarvi E., Tyystjarvi T. Oxidative stress and photoinhibition can be separated in the cyanobacterium Synechocystissp. PCC 6803. Biochim. Biophys. Acta. 2014;1837(2):217–225. doi: 10.1016/j.bbabio.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Blagosklonny M.V. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5(1):13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 83.Sepehripour S., Dhaliwal K., Dheansa B. Hyperbaric oxygen therapy and intermittent ischaemia in the treatment of chronic wounds. Int. Wound J. 2018;15(2):310. doi: 10.1111/iwj.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maharjan S., Alva J., Cámara C., Rubio A.G., Hernández D., Delavaux C., Correa E., Romo M.D., Bonilla D., Santiago M.L., Li W., Cheng F., Ying G., Zhang Y.S. Symbiotic photosynthetic oxygenation within 3D-bioprinted vascularized tissues. Matter. 2021;4(1):217–240. doi: 10.1016/j.matt.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pencik O., Molnarova K., Durdakova M., Kolackova M., Klofac D., Kucsera A., Capal P., Svec P., Bytesnikova Z., Richtera L., Brtnicky M., Adam V., Huska D. Not so dangerous? PET microplastics toxicity on freshwater microalgae and cyanobacteria. Environ. Pollut. 2023;329 doi: 10.1016/j.envpol.2023.121628. [DOI] [PubMed] [Google Scholar]
- 86.Liang F., Lindblad P. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium SynechocystisPCC 6803. Metab. Eng. 2016;38:56–64. doi: 10.1016/j.ymben.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 87.De Porcellinis A.J., Norgaard H., Brey L.M.F., Erstad S.M., Jones P.R., Heazlewood J.L., Sakuragi Y. Overexpression of bifunctional fructose-1,6-bisphosphatase/sedoheptulose-1,7-bisphosphatase leads to enhanced photosynthesis and global reprogramming of carbon metabolism in Synechococcus sp. PCC 7002. Metab. Eng. 2018;47:170–183. doi: 10.1016/j.ymben.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Kedzior M., Kacar B. Quantification of RuBisCO expression and photosynthetic oxygen evolution in cyanobacteria. Bio Protoc. 2021;11(20) doi: 10.21769/BioProtoc.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moreira J.B., Vaz B.d.S., Cardias B.B., Cruz C.G., Almeida A.C.A.d., Costa J.A.V., Morais M.G.d. Microalgae polysaccharides: an alternative source for food production and sustainable agriculture. Polysaccharides. 2022;3(2):441–457. doi: 10.3390/polysaccharides3020027. [DOI] [Google Scholar]
- 90.Tischer R.G., Moore B.G. An extracellular polysaccharide produced by Palmella mucosa Kütz. Arch. Mikrobiol. 1964;49:158–166. doi: 10.1007/bf00422139. [DOI] [PubMed] [Google Scholar]
- 91.Debnath S., Muthuraj M., Bandyopadhyay T.K., Bobby M.N., Vanitha K., Tiwari O.N., Bhunia B. Engineering strategies and applications of cyanobacterial exopolysaccharides: a review on past achievements and recent perspectives. Carbohydr. Polym. 2024;328 doi: 10.1016/j.carbpol.2023.121686. [DOI] [PubMed] [Google Scholar]
- 92.Uhliariková I., Chválová B., Matulová M., Cepák V., Lukavský J., Capek P. Extracellular biopolymers produced by freshwater cyanobacteria: a screening study. Chem. Pap. 2019;73(3):771–776. doi: 10.1007/s11696-018-0625-1. [DOI] [Google Scholar]
- 93.Cruz J.D., Delattre C., Felpeto A.B., Pereira H., Pierre G., Morais J., Petit E., Silva J., Azevedo J., Elboutachfaiti R., Maia I.B., Dubessay P., Michaud P., Vasconcelos V. Bioprospecting for industrially relevant exopolysaccharide-producing cyanobacteria under Portuguese simulated climate. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-40542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohd Nadzir M., Nurhayati R.W., Idris F.N., Nguyen M.H. Biomedical applications of bacterial exopolysaccharides: a review. Polymers. 2021;13(4) doi: 10.3390/polym13040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahana T.G., Rekha P.D. A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int. J. Biol. Macromol. 2019;131:10–18. doi: 10.1016/j.ijbiomac.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 96.Niknezhad S.V., Mehrali M., Khorasgani F.R., Heidari R., Kadumudi F.B., Golafshan N., Castilho M., Pennisi C.P., Hasany M., Jahanshahi M., Mehrali M., Ghasemi Y., Azarpira N., Andresen T.L., Dolatshahi-Pirouz A. Enhancing volumetric muscle loss (VML) recovery in a rat model using super durable hydrogels derived from bacteria. Bioact. Mater. 2024;38:540–558. doi: 10.1016/j.bioactmat.2024.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Falsini S., Rosi M.C., Ravegnini E., Schiff S., Gonnelli C., Papini A., Adessi A., Urciuoli S., Ristori S. Nanoformulations with exopolysaccharides from cyanobacteria: enhancing the efficacy of bioactive molecules in the Mediterranean fruit fly control. Environ. Sci. Pollut. Control Ser. 2023;30(35):83760–83770. doi: 10.1007/s11356-023-28180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biller S.J., Munoz-Marin M.D.C., Lima S., Matinha-Cardoso J., Tamagnini P., Oliveira P. Isolation and characterization of cyanobacterial extracellular vesicles. J. Vis. Exp. 2022;180 doi: 10.3791/63481. [DOI] [PubMed] [Google Scholar]
- 99.Toyofuku M., Nomura N., Eberl L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019;17(1):13–24. doi: 10.1038/s41579-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 100.Biller S.J., Schubotz F., Roggensack S.E., Thompson A.W., Summons R.E., Chisholm S.W. Bacterial vesicles in marine ecosystems. Science. 2014;343(6167):183–186. doi: 10.1126/science.1243457. [DOI] [PubMed] [Google Scholar]
- 101.Pardo Y.A., Florez C., Baker K.M., Schertzer J.W., Mahler G.J. Detection of outer membrane vesicles in Synechocystis PCC 6803. FEMS Microbiol. Lett. 2015;362(20) doi: 10.1093/femsle/fnv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu Y., Tiago Guerra L., Li Z., Ludwig M., Charles Dismukes G., Bryant D.A. Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC 7002: cell factories for soluble sugars. Metab. Eng. 2013;16:56–67. doi: 10.1016/j.ymben.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Yin H., Chen C.Y., Liu Y.W., Tan Y.J., Deng Z.L., Yang F., Huang F.Y., Wen C., Rao S.S., Luo M.J., Hu X.K., Liu Z.Z., Wang Z.X., Cao J., Liu H.M., Liu J.H., Yue T., Tang S.Y., Xie H. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9(9):2678–2693. doi: 10.7150/thno.31884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mota R., Vidal R., Pandeirada C., Flores C., Adessi A., De Philippis R., Nunes C., Coimbra M.A., Tamagnini P. Cyanoflan: a cyanobacterial sulfated carbohydrate polymer with emulsifying properties. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115525. [DOI] [PubMed] [Google Scholar]
- 105.Brito A., Ramos V., Mota R., Lima S., Santos A., Vieira J., Vieira C.P., Kastovsky J., Vasconcelos V.M., Tamagnini P. Description of new genera and species of marine cyanobacteria from the Portuguese Atlantic coast. Mol. Phylogenet. Evol. 2017;111:18–34. doi: 10.1016/j.ympev.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 106.Oliveira P., Martins N., Santos M., Couto N., Wright P., Tamagnini P. The Anabaenasp. PCC 7120 exoproteome: taking a peek outside the box. Life. 2015;5(1):130–163. doi: 10.3390/life5010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zarantonello V., Silva T.P., Noyma N.P., Gamalier J.P., Mello M.M., Marinho M.M., Melo R.C.N. The cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) responds to environmental stresses with increased vesiculation detected at single-cell resolution. Front. Microbiol. 2018;9:272. doi: 10.3389/fmicb.2018.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng W., Bergman B., Chen B., Zheng S., Xiang G., Rasmussen U. Cellular responses in the cyanobacterial symbiont during its vertical transfer between plant generations in the Azolla microphylla symbiosis. New Phytol. 2009;181(1):53–61. doi: 10.1111/j.1469-8137.2008.02644.x. [DOI] [PubMed] [Google Scholar]
- 109.Cui J., Xie Y., Sun T., Chen L., Zhang W. Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.144111. [DOI] [PubMed] [Google Scholar]
- 110.Ayangbenro A., Babalola O. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int. J. Environ. Res. Publ. Health. 2017;14(1) doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng S.Y., Show P.L., Lau B.F., Chang J.S., Ling T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019;37(11):1255–1268. doi: 10.1016/j.tibtech.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 112.Singh J.S., Kumar A., Rai A.N., Singh D.P. Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016;7:529. doi: 10.3389/fmicb.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Al-Homaidan A.A., Alabdullatif J.A., Al-Hazzani A.A., Al-Ghanayem A.A., Alabbad A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015;22(6):795–800. doi: 10.1016/j.sjbs.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kwak H.W., Kim M.K., Lee J.Y., Yun H., Kim M.H., Park Y.H., Lee K.H. Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium (VI) removal. Algal Res. 2015;7:92–99. doi: 10.1016/j.algal.2014.12.006. [DOI] [Google Scholar]
- 115.Leong Y.K., Chang J.S. Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour. Technol. 2020;303 doi: 10.1016/j.biortech.2020.122886. [DOI] [PubMed] [Google Scholar]
- 116.Tiwari S., Parihar P., Patel A., Singh R., Prasad S.M. Metals in cyanobacteria: physiological and molecular regulation. Cyanobacteria. 2019:261–276. doi: 10.1016/b978-0-12-814667-5.00013-1. [DOI] [Google Scholar]
- 117.K K.R., Sardar U.R., Bhargavi E., Devi I., Bhunia B., Tiwari O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: a critical review. Carbohydr. Polym. 2018;199:353–364. doi: 10.1016/j.carbpol.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 118.Ozturk S., Aslim B., Suludere Z., Tan S. Metal removal of cyanobacterial exopolysaccharides by uronic acid content and monosaccharide composition. Carbohydr. Polym. 2014;101:265–271. doi: 10.1016/j.carbpol.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 119.Shen L., Li Z., Wang J., Liu A., Li Z., Yu R., Wu X., Liu Y., Li J., Zeng W. Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd(II) by Synechocystissp. PCC6803. Environ. Sci. Pollut. Res. Int. 2018;25(21):20713–20722. doi: 10.1007/s11356-018-2163-3. [DOI] [PubMed] [Google Scholar]
- 120.Mejáre M., Bülow L. Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol. 2001;19(2):67–73. doi: 10.1016/S0167-7799(00)01534-1. [DOI] [PubMed] [Google Scholar]
- 121.Swift D.T., Forciniti D. Accumulation of lead by Anabaena cylindrica : mathematical modeling and an energy dispersive X-ray study. Biotechnol. Bioeng. 1997;55(2):408–418. doi: 10.1100/2012/179782. [DOI] [PubMed] [Google Scholar]
- 122.Cobbett C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123(3):825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Case A.E., Atsumi S. Cyanobacterial chemical production. J. Biotechnol. 2016;231:106–114. doi: 10.1016/j.jbiotec.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 124.Gao X., Sun T., Pei G., Chen L., Zhang W. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 2016;100(8):3401–3413. doi: 10.1007/s00253-016-7374-2. [DOI] [PubMed] [Google Scholar]
- 125.Sun T., Li S., Song X., Diao J., Chen L., Zhang W. Toolboxes for cyanobacteria: recent advances and future direction. Biotechnol. Adv. 2018;36(4):1293–1307. doi: 10.1016/j.biotechadv.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Hilzinger J.M., Friedline S., Sivanandan D., Cheng Y.-F., Yamazaki S., Clark D.S., Skerker J.M., Arkin A.P. Acetaminophen production in the edible, filamentous cyanobacterium Arthrospira platensis. bioRxiv preprint. 2022 doi: 10.1101/2022.06.30.498297. [DOI] [Google Scholar]
- 127.Shah M.M., Liang Y., Cheng J.J., Daroch M. Astaxanthin-producing green microalga haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci. 2016;7:531. doi: 10.3389/fpls.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diao J., Song X., Zhang L., Cui J., Chen L., Zhang W. Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin. Metab. Eng. 2020;61:275–287. doi: 10.1016/j.ymben.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 129.Wang X., Chen L., Liu J., Sun T., Zhang W. Light-driven biosynthesis of myo-inositol directly from CO2 in Synechocystis sp. PCC 6803. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.566117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun T., Li Z., Li S., Chen L., Zhang W. Exploring and validating key factors limiting cyanobacteria-based CO2 bioconversion: case study to maximize myo-inositol biosynthesis. Chem. Eng. J. 2023;452 doi: 10.1016/j.cej.2022.139158. [DOI] [Google Scholar]
- 131.Santos-Merino M., Yun L., Ducat D.C. Cyanobacteria as cell factories for the photosynthetic production of sucrose. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1126032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baunach M., Guljamow A., Miguel-Gordo M., Dittmann E. Harnessing the potential: advances in cyanobacterial natural product research and biotechnology. Nat. Prod. Rep. 2023 doi: 10.1039/d3np00045a. [DOI] [PubMed] [Google Scholar]
- 133.Uzair B., Tabassum S., Rasheed M., Rehman S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012 doi: 10.1100/2012/179782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khalifa S.A.M., Shedid E.S., Saied E.M., Jassbi A.R., Jamebozorgi F.H., Rateb M.E., Du M., Abdel-Daim M.M., Kai G.Y., Al-Hammady M.A.M., Xiao J., Guo Z., El-Seedi H.R. Cyanobacteria-from the oceans to the potential biotechnological and biomedical applications. Mar. Drugs. 2021;19(5) doi: 10.3390/md19050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Demay J., Bernard C., Reinhardt A., Marie B. Natural products from cyanobacteria: focus on beneficial activities. Mar. Drugs. 2019;17(6) doi: 10.3390/md17060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sijtsma L., de Swaaf M.E. Biotechnological production and applications of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004;64(2):146–153. doi: 10.1007/s00253-003-1525-y. [DOI] [PubMed] [Google Scholar]
- 137.Hassan S., Meenatchi R., Pachillu K., Bansal S., Brindangnanam P., Arockiaraj J., Kiran G.S., Selvin J. Identification and characterization of the novel bioactive compounds from microalgae and cyanobacteria for pharmaceutical and nutraceutical applications. J. Basic Microbiol. 2022;62(9):999–1029. doi: 10.1002/jobm.202100477. [DOI] [PubMed] [Google Scholar]
- 138.Perera R., Herath K., Sanjeewa K.K.A., Jayawardena T.U. Recent reports on bioactive compounds from marine cyanobacteria in relation to human health applications. Life. 2023;13(6) doi: 10.3390/life13061411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwartz J.L., Shklar G. A cyanobacteria extract and β‐carotene stimulate an antitumor host response against an oral cancer cell line. Phytother Res. 1989;3(6):243–248. doi: 10.1002/ptr.2650030605. [DOI] [Google Scholar]
- 140.Gdara N.B., Belgacem A., Khemiri I., Mannai S., Bitri L. Protective effects of phycocyanin on ischemia/reperfusion liver injuries. Biomed. Pharmacother. 2018;102:196–202. doi: 10.1016/j.biopha.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 141.Saini M.K., Vaiphei K., Sanyal S.N. Chemoprevention of DMH-induced rat colon carcinoma initiation by combination administration of piroxicam and C-phycocyanin. Mol. Cell. Biochem. 2012;361(1–2):217–228. doi: 10.1007/s11010-011-1106-9. [DOI] [PubMed] [Google Scholar]
- 142.Zhu C., Ling Q., Cai Z., Wang Y., Zhang Y., Hoffmann P.R., Zheng W., Zhou T., Huang Z. Selenium-containing phycocyanin from Se-enriched spirulina platensis reduces inflammation in dextran sulfate sodium-induced colitis by inhibiting NF-kappaB activation. J. Agric. Food Chem. 2016;64(24):5060–5070. doi: 10.1021/acs.jafc.6b01308. [DOI] [PubMed] [Google Scholar]
- 143.Mahmoud Huheihel V.I. Jacov tal, shoshana (malis) arad, activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods. 2002;50(2–3):189–200. doi: 10.1016/s0165-022x(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 144.Zampieri R.M., Adessi A., Caldara F., Codato A., Furlan M., Rampazzo C., De Philippis R., La Rocca N., Dalla Valle L. Anti-inflammatory activity of exopolysaccharides from Phormidium sp. ETS05, the most abundant cyanobacterium of the therapeutic euganean thermal muds, using the zebrafish model. Biomolecules. 2020;10(4) doi: 10.3390/biom10040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matinha-Cardoso J., Coutinho F., Lima S., Eufrasio A., Carvalho A.P., Oliva-Teles A., Bessa J., Tamagnini P., Serra C.R., Oliveira P. Novel protein carrier system based on cyanobacterial nano-sized extracellular vesicles for application in fish. Microb. Biotechnol. 2022;15(8):2191–2207. doi: 10.1111/1751-7915.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li W., Zhong D., Hua S., Du Z., Zhou M. Biomineralized biohybrid algae for tumor hypoxia modulation and cascade radio-photodynamic therapy. ACS Appl. Mater. Interfaces. 2020;12(40):44541–44553. doi: 10.1021/acsami.0c14400. [DOI] [PubMed] [Google Scholar]
- 147.Zhong D., Li W., Hua S., Qi Y., Xie T., Qiao Y., Zhou M. Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy. Theranostics. 2021;11(8):3580–3594. doi: 10.7150/thno.55441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen J.E., Goldstone A.B., Paulsen M.J., Shudo Y., Steele A.N., Edwards B.B., Patel J.B., MacArthur J.W., Jr H., S M., Burnett C.E., Jaatinen K.J., Thakore A.D., Farry J.M., Truong V.N., Bourdillon A.T., Stapleton L.M., Eskandari A., Fairman A.S., Hiesinger W., Esipova T.V., Patrick W.L., Ji K., Shizuru J.A., Woo Y.J. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci. Adv. 2017;3(6) doi: 10.1126/sciadv.1603078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guo M., Wang S., Guo Q., Hou B., Yue T., Ming D., Zheng B. NIR-responsive spatiotemporally controlled cyanobacteria micro-nanodevice for intensity-modulated chemotherapeutics in rheumatoid arthritis. ACS Appl. Mater. Interfaces. 2021;13(16):18423–18431. doi: 10.1021/acsami.0c20514. [DOI] [PubMed] [Google Scholar]
- 150.Wang B., Zhou L., Guo Y., Guo H., Zhong Y., Huang X., Ge Y., Wang Q., Chu X., Jin Y., Lan K., Yang M., Qu J. Cyanobacteria-based self-oxygenated photodynamic therapy for anaerobic infection treatment and tissue repair. Bioact. Mater. 2022;12:314–326. doi: 10.1016/j.bioactmat.2021.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ozugur S., Chavez M.N., Sanchez-Gonzalez R., Kunz L., Nickelsen J., Straka H. Green oxygen power plants in the brain rescue neuronal activity. iScience. 2021;24(10) doi: 10.1016/j.isci.2021.103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X., Yang C., Yu Y., Zhao Y. In situ 3D bioprinting living photosynthetic scaffolds for autotrophic wound healing. Research. 2022;2022 doi: 10.34133/2022/9794745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu C., Ye Q., Hua S., Huang H., Zhong D., Liang F., Zhou M. Microalgae-based natural oral hydrogel system for synergistic treatment of lead poisoning-related diseases. Nano Today. 2023;53 doi: 10.1016/j.nantod.2023.102034. [DOI] [Google Scholar]
- 154.Li L., Yang C., Ma B., Lu S., Liu J., Pan Y., Wang X., Zhang Y., Wang H., Sun T., Liu D. Hydrogel-encapsulated engineered microbial consortium as a photoautotrophic "living material" for promoting skin wound healing. ACS Appl. Mater. Interfaces. 2023;15(5):6536–6547. doi: 10.1021/acsami.2c20399. [DOI] [PubMed] [Google Scholar]
- 155.Hu H., Zhong D., Li W., Lin X., He J., Sun Y., Wu Y., Shi M., Chen X., Xu F., Zhou M. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022;42 doi: 10.1016/j.nantod.2021.101368. [DOI] [Google Scholar]
- 156.Zhong D., Zhang D., Xie T., Zhou M. Biodegradable microalgae-based carriers for targeted delivery and imaging-guided therapy toward lung metastasis of breast cancer. Small. 2020;16(20) doi: 10.1002/smll.202000819. [DOI] [PubMed] [Google Scholar]
- 157.Zhang D., He J., Cui J., Wang R., Tang Z., Yu H., Zhou M. Oral microalgae-nano integrated system against radiation-induced injury. ACS Nano. 2023;17(11):10560–10576. doi: 10.1021/acsnano.3c01502. [DOI] [PubMed] [Google Scholar]
- 158.Liu R., Qin S., Li W. Phycocyanin: anti-inflammatory effect and mechanism. Biomed. Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113362. [DOI] [PubMed] [Google Scholar]
- 159.Burja A.M., Banaigs B., Abou-Mansour E., Burgess J.G., Wright P.C. Marine cyanobacteria—a prolific source of natural products. Tetrahedron. 2001;57(46):9347–9377. doi: 10.1016/S0040-4020(01)00931-0. [DOI] [Google Scholar]
- 160.Jing X., Yang F., Shao C., Wei K., Xie M., Shen H., Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer. 2019;18(1):157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu S., Zhu X., Zhang C., Huang W., Zhou Y., Yan D. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Commun. 2018;9(1):2053. doi: 10.1038/s41467-018-04318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou T.-J., Xing L., Fan Y.-T., Cui P.-F., Jiang H.-L. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J. Contr. Release. 2019;307:44–54. doi: 10.1016/j.jconrel.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 163.Sun T., Zhang Y., Zhang C., Wang H., Pan H., Liu J., Li Z., Chen L., Chang J., Zhang W. Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy. Front. Bioeng. Biotechnol. 2020;8:237. doi: 10.3389/fbioe.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stapleton L.M., Farry J.M., Lucian H.J., Wang H., Totherow K.P., Paulsen M.J., Agmon G., Cabatu M., Thakore A.D., Eskandari A.N., Anilkumar S.D., Buncom F.J., Appel E.A., Woo Y.J. Cyanobacteria-alginate microgels for sustained photosynthetic oxygen delivery to rescue cardiomyocytes in an ischemic milieu. Circulation. 2019;140 doi: 10.1161/circ.140.suppl_1.15828. [DOI] [Google Scholar]
- 165.Ahmed A.A.Q., Besio R., Xiao L., Forlino A. Outer membrane vesicles (OMVs) as biomedical tools and their relevance as immune-modulating agents against H. pylori infections: current status and future prospects. Int. J. Mol. Sci. 2023;24(10) doi: 10.3390/ijms24108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aytar Celik P., Erdogan-Gover K., Barut D., Enuh B.M., Amasya G., Sengel-Turk C.T., Derkus B., Cabuk A. Bacterial membrane vesicles as smart drug delivery and carrier systems: a new nanosystems tool for current anticancer and antimicrobial therapy. Pharmaceutics. 2023;15(4) doi: 10.3390/pharmaceutics15041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Durai P., Batool M., Choi S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs. 2015;13(7):4217–4230. doi: 10.3390/md13074217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Snyder D.S., Brahamsha B., Azadi P., Palenik B. Structure of compositionally simple lipopolysaccharide from marine Synechococcus. J. Bacteriol. 2009;191(17):5499–5509. doi: 10.1128/JB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Song P., Xu D., Yue J., Ma Y., Dong S., Feng J. Recent advances in soil remediation technology for heavy metal contaminated sites: a critical review. Sci. Total Environ. 2022;838(Pt 3) doi: 10.1016/j.scitotenv.2022.156417. [DOI] [PubMed] [Google Scholar]
- 170.Almomani F., Bhosale R.R. Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: application of isotherm, kinetic models and process optimization. Sci. Total Environ. 2021;755(Pt 2) doi: 10.1016/j.scitotenv.2020.142654. [DOI] [PubMed] [Google Scholar]
- 171.Thore E.S.J., Muylaert K., Bertram M.G., Brodin T. Microalgae. Curr. Biol. 2023;33(3):R91–R95. doi: 10.1016/j.cub.2022.12.032. [DOI] [PubMed] [Google Scholar]
- 172.Tao S., Huaishu H., Yingying Z., Yaru X., Yize L., Kungang P., Fenfang Z., Jing L., Yindong T., Weiwen Z., Lei C. Engineered cyanobacteria-based living materials for bioremediation of heavy metals both in vitro and in vivo. ACS Nano. 2024;18(27):17694–17706. doi: 10.1021/acsnano.4c02493. [DOI] [PubMed] [Google Scholar]
- 173.Massot F., Bernard N., Alvarez L.M.M., Martorell M.M., Mac Cormack W.P., Ruberto L.A.M. Microbial associations for bioremediation. What does "microbial consortia" mean? Appl. Microbiol. Biotechnol. 2022;106(7):2283–2297. doi: 10.1007/s00253-022-11864-8. [DOI] [PubMed] [Google Scholar]
- 174.Liao M.J., Din M.O., Tsimring L., Hasty J. Rock-paper-scissors Engineered population dynamics increase genetic stability. Science. 2019;365(6457):1045–1049. doi: 10.1126/science.aaw0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lawson C.E., Harcombe W.R., Hatzenpichler R., Lindemann S.R., Loffler F.E., O'Malley M.A., Garcia Martin H., Pfleger B.F., Raskin L., Venturelli O.S., Weissbrodt D.G., Noguera D.R., McMahon K.D. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 2019;17(12):725–741. doi: 10.1038/s41579-019-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith M.J., Francis M.B. A designed A. vinelandii-S. Elongatus coculture for chemical photoproduction from air, water, phosphate, and trace metals. ACS Synth. Biol. 2016;5(9):955–961. doi: 10.1021/acssynbio.6b00107. [DOI] [PubMed] [Google Scholar]
- 177.Perera I.A., Abinandan S., Subashchandrabose S.R., Venkateswarlu K., Naidu R., Megharaj M. Advances in the technologies for studying consortia of bacteria and cyanobacteria/microalgae in wastewaters. Crit. Rev. Biotechnol. 2019;39(5):709–731. doi: 10.1080/07388551.2019.1597828. [DOI] [PubMed] [Google Scholar]
- 178.Fedeson D.T., Saake P., Calero P., Nikel P.I., Ducat D.C. Biotransformation of 2,4-dinitrotoluene in a phototrophic co-culture of engineered Synechococcus elongatus and Pseudomonas putida. Microb. Biotechnol. 2020;13(4):997–1011. doi: 10.1111/1751-7915.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li T., Li C.T., Butler K., Hays S.G., Guarnieri M.T., Oyler G.A., Betenbaugh M.J. Mimicking lichens: incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform. Biotechnol. Biofuels. 2017;10:55. doi: 10.1186/s13068-017-0736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhu H., Xu L., Luan G., Zhan T., Kang Z., Li C., Lu X., Zhang X., Zhu Z., Zhang Y., Li Y. A miniaturized bionic ocean-battery mimicking the structure of marine microbial ecosystems. Nat. Commun. 2022;13(1):5608. doi: 10.1038/s41467-022-33358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bariana M., Aw M.S., Kurkuri M., Losic D. Tuning drug loading and release properties of diatom silica microparticles by surface modifications. Int. J. Pharm. 2013;443(1–2):230–241. doi: 10.1016/j.ijpharm.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 182.Uthappa U.T., Brahmkhatri V., Sriram G., Jung H.Y., Yu J., Kurkuri N., Aminabhavi T.M., Altalhi T., Neelgund G.M., Kurkuri M.D. Nature engineered diatom biosilica as drug delivery systems. J. Contr. Release. 2018;281:70–83. doi: 10.1016/j.jconrel.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 183.Xie S., Jiao N., Tung S., Liu L. Controlled regular locomotion of algae cell microrobots. Biomed. Microdevices. 2016;18(3):47. doi: 10.1007/s10544-016-0074-y. [DOI] [PubMed] [Google Scholar]
- 184.Shchelik I.S., Sieber S., Gademann K. Green algae as a drug delivery system for the controlled release of antibiotics. Chemistry. 2020;26(70):16644–16648. doi: 10.1002/chem.202003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Srivastava R., Prajapati R., Kanda T., Yadav S., Singh N., Yadav S., Mishra R., Atri N. Phycochemistry and bioactivity of cyanobacterial secondary metabolites. Mol. Biol. Rep. 2022;49(11):11149–11167. doi: 10.1007/s11033-022-07911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stevens M., Peigneur S., Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011;2:71. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang K.C., Chen Z., Jiang Y., Akare S., Kolber-Simonds D., Condon K., Agoulnik S., Tendyke K., Shen Y., Wu K.M., Mathieu S., Choi H.W., Zhu X., Shimizu H., Kotake Y., Gerwick W.H., Uenaka T., Woodall-Jappe M., Nomoto K. Apratoxin A shows novel pancreas-targeting activity through the binding of sec 61. Mol. Cancer Therapeut. 2016;15(6):1208–1216. doi: 10.1158/1535-7163.MCT-15-0648. [DOI] [PubMed] [Google Scholar]
- 188.Weiss C., Figueras E., Borbely A.N., Sewald N. Cryptophycins: cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017;23(7–8):514–531. doi: 10.1002/psc.3015. [DOI] [PubMed] [Google Scholar]
- 189.Angermayr S.A., Gorchs Rovira A., Hellingwerf K.J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015;33(6):352–361. doi: 10.1016/j.tibtech.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 190.Zhang X., Zhang Y., Zhang C., Yang C., Tian R., Sun T., Zhang W., Chang J., Wang H. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy. Colloids Surf. B Biointerfaces. 2021;201 doi: 10.1016/j.colsurfb.2021.111640. [DOI] [PubMed] [Google Scholar]
- 191.Zhang X., Pang G., Sun T., Liu X., Pan H., Zhang Y., Liu J., Chang J., Wang H., Liu D. A red light-controlled probiotic bio-system for in-situ gut-brain axis regulation. Biomaterials. 2023;294 doi: 10.1016/j.biomaterials.2023.122005. [DOI] [PubMed] [Google Scholar]
- 192.Wang J., Su Q., Lv Q., Cai B., Xiaohalati X., Wang G., Wang Z., Wang L. Oxygen-generating cyanobacteria powered by upconversion-nanoparticles-converted near-infrared light for ischemic stroke treatment. Nano Lett. 2021;21(11):4654–4665. doi: 10.1021/acs.nanolett.1c00719. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y., Zhong D., He Y., Jiang J., Xie W., Tang Z., Qiu J., Luo J., Wang X. Photoresponsive hydrogel-coated upconversion cyanobacteria nanocapsules for myocardial infarction prevention and treatment. Adv. Sci. 2022;9(30) doi: 10.1002/advs.202202920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ebrahimkhani M.R., Ebisuya M. Synthetic developmental biology: build and control multicellular systems. Curr. Opin. Chem. Biol. 2019;52:9–15. doi: 10.1016/j.cbpa.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 195.Liu X., Tang T.C., Tham E., Yuk H., Lin S., Lu T.K., Zhao X. Stretchable living materials and devices with hydrogel-elastomer hybrids hosting programmed cells. Proc. Natl. Acad. Sci. U.S.A. 2017;114(9):2200–2205. doi: 10.1073/pnas.1618307114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li J., Zhao H., Zheng L., An W. Advances in synthetic biology and biosafety governance. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.598087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Whitford C.M., Dymek S., Kerkhoff D., Marz C., Schmidt O., Edich M., Droste J., Pucker B., Ruckert C., Kalinowski J. Auxotrophy to Xeno-DNA: an exploration of combinatorial mechanisms for a high-fidelity biosafety system for synthetic biology applications. J. Biol. Eng. 2018;12:13. doi: 10.1186/s13036-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choi W.Y., Lee W.K., Kim T.H., Ryu Y.K., Park A., Lee Y.J., Heo S.J., Oh C., Chung Y.C., Kang D.H. The effects of spirulina maxima extract on memory improvement in those with mild cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2022;14(18) doi: 10.3390/nu14183714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prete V., Abate A.C., Di Pietro P., De Lucia M., Vecchione C., Carrizzo A. Beneficial effects of spirulina supplementation in the management of cardiovascular diseases. Nutrients. 2024;16(5) doi: 10.3390/nu16050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Karizi S.r., Armanmehr F., Azadi H.G., Zahroodi H.S., Ghalibaf A.M., Bazzaz B.S.F., Abbaspour M., Boskabadi J., Eslami S., Taherzadeh Z. A randomized, double-blind placebo-controlled add-on trial to assess the efficacy, safety, and anti-atherogenic effect of Spirulina platensis in patients with inadequately controlled type 2 diabetes mellitus. Phytother Res. 2023;37(4):1435–1448. doi: 10.1002/ptr.7674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.