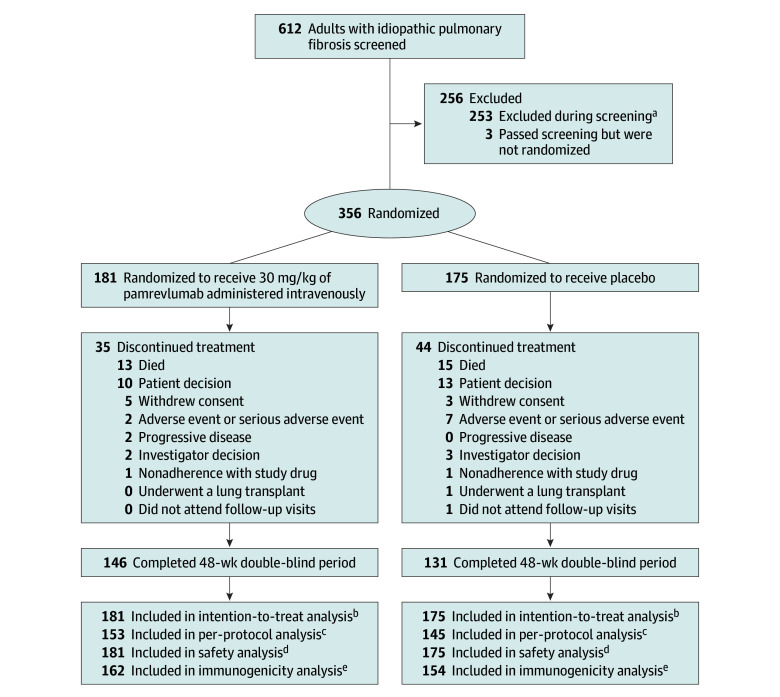
Figure 1. Patient Randomization and Follow-Up.
aThe most common reasons were (1) percent predicted forced vital capacity values did not meet inclusion criteria at screening and on day 1, (2) did not have a guideline-based diagnosis of idiopathic pulmonary fibrosis, (3) did not have a diagnosis of idiopathic pulmonary fibrosis confirmed by high-resolution computed tomography imaging scan, (4) had percent predicted values for diffusing capacity of the lungs for carbon monoxide that did not meet inclusion criteria, and (5) had evidence of significant obstructive lung disease.
bIncluded all randomized patients during the 48-week study.
cIncluded all randomized patients (1) who completed at least 36 weeks of treatment with a pulmonary function test assessment at baseline and at least once after baseline and (2) without major protocol deviations that significantly affected the efficacy analyses.
dIncluded all patients who received at least 1 dose of study drug.
eIncluded all patients who were in the safety population and who had at least 1 evaluable immunogenicity assessment after baseline.