FIG. 9.
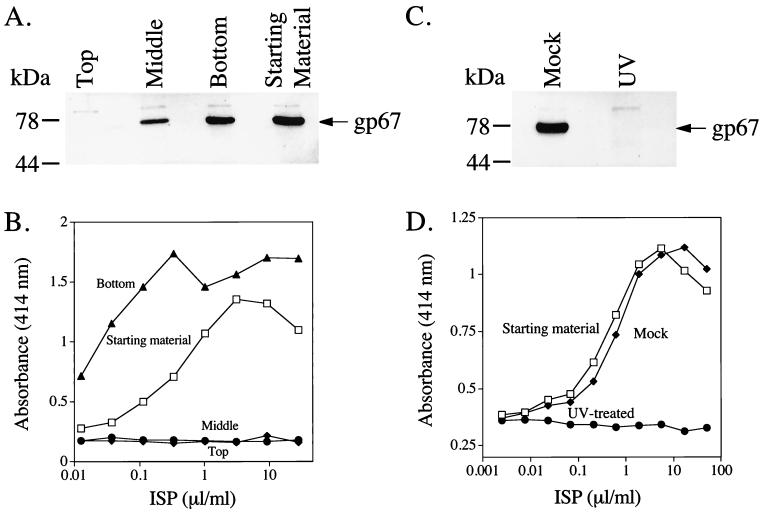
The ISP requires live baculovirus for its IFN-stimulating activity. (A) One milliliter of the ISP was subjected to ultracentrifugation at 100,000 × g for 3 h at 4°C. The ISP was removed in three fractions: top, middle, and bottom. These fractions were then used to infect Sf9 cells. Sf9 cells (6.5 × 104/well) were exposed to the ISP fractions for 1 h and then washed thoroughly. After 5 days at 27°C, the media were removed and infected cells were lysed. Cleared lysates (20 μl) were separated on SDS–4 to 20% polyacrylamide gels and evidence of active baculovirus infection was detected by Western blotting with the AcV5 antibody specific for gp67. (B) Portions of the fractions were also added to 1.5 × 105 RAW264.7 cells/well (75-μl volume) in 96-well flat-bottom plates. After 8 h at 37°C, 100 μl of the supernatant was removed for IFN-α/β ELISA. (C) The ISP (125 μl) was placed in a sterile petri dish in a laminar flow hood and subjected to either shortwave UV light for 25 min at a distance of 7 cm or subjected to normal fluorescent light for the same period of time. The two preparations were then used to infect Sf9 cells (C) as above or treat RAW cells for IFN production (D) as above.