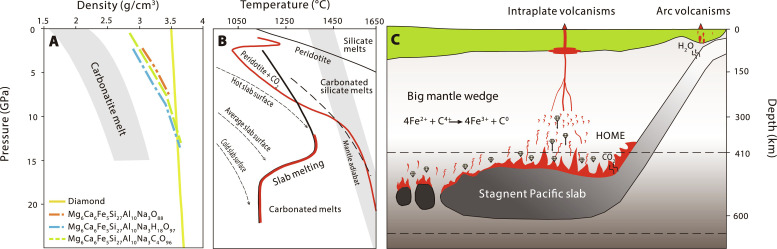
Fig. 6. Formation mechanism of HOME.
(A) Density-pressure profiles of melts and diamond. Solid yellow: diamond; dashed orange: dry MORB (Mg6Ca6Fe5Si27Al10Na3O88): dashed green: MORB with 4.9 wt % H2O (Mg6Ca6Fe5Si27Al10Na3H18O97); dashed blue (Mg6Ca6Fe5Si27Al10Na3C4O96): MORB with 5.3 wt % CO2. Data are reported in table S6. The density-pressure profiles of a variety of carbonate melts are from Massuyeau et al. (75). (B) The melting curves of carbonated MORB and carbonated peridotites compared to subduction geotherms and ambient mantle adiabat, modified from Thomson et al. (42) and Dasgupta (76). (C) Cartoon showing the formation of HOME in the BMW. Low-degree melting of stagnant carbonated Pacific slab generates carbonated melts. With excess C4+ replenished from subducted slabs, carbon-iron redox reaction happened in the melts (4Fe2+ + C4+ ➔ 4Fe3+ + C0), and efficient separation of the newly formed diamond from the melts due to density difference would have elevated the melts’ Fe3+/∑Fe, forming the HOME. These melts have HIMU elemental signatures, which subsequently metasomatizes the ambient peridotites. The interaction would have promoted carbon-iron redox reaction to precipitate diamond from melts and meanwhile elevate the Fe3+/∑Fe of carbonated peridotites. Excess CO2 from the subducted slab-derived carbonated melts decreases the solidus of metasomatized peridotites, leading to partial melting of the high-Fe3+/∑Fe carbonate-metasomatized peridotitic domains to produce melts inherited the HOME signature.