Sir,
The coronavirus disease 2019 (COVID-19) vaccination has been rapidly implemented worldwide. Local reactions, such as erythema, induration and pain at the injection site, are among the most common side effects, whereas delayed injection-site reactions have been rarely reported.[1]
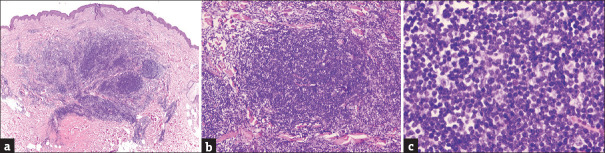
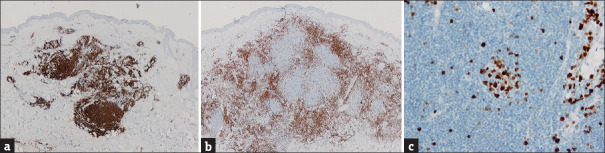
A 51-year-old healthy woman presented with a 4-month history of an asymptomatic nodule on her left arm. The lesions had appeared in the same place where the second dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine (Pfizer-BioNTech) had been injected a month earlier. Physical examination showed a 1 cm reddish elastic nodule [Figure 1a]. The dermoscopic evaluation revealed a lesion with pinkish background and unfocussed arborizing vessels [Figure 1b]. Surgical excision was performed. Histological examination demonstrated large nodular infiltrates of small size, non-atypical, lymphocytes with follicle formation, extending from the superficial to the deep dermis without epidermal involvement [Figure 2]. Immunohistochemistry revealed a mixture of B and T cells, highlighted by cluster of differentiate 20 (CD20) and CD3 stains [Figure 3]. Based on clinical and histological findings, the diagnosis of cutaneous pseudolymphoma (CPL) associated with the SARS-CoV-2 vaccine injection was established. Sixteen-month control revealed neither signs of recurrence nor the development of new lesions after successive doses of the vaccine.
Figure 1.
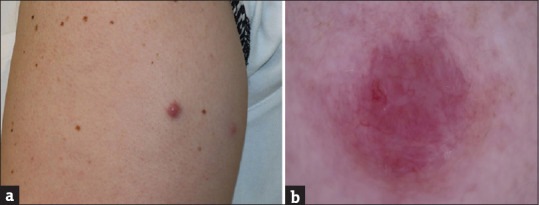
Clinical and dermoscopic findings. (a). Physical examination: a reddish, elastic to touch and well-demarcated nodule, with a rounded and regular outline. (b). Dermoscopic evaluation: multiple non-focused, non-fine vessels on a pinkish erythematous background, with no other specific findings
Figure 2.
(a) A benign nodular lymphoid proliferation extending from the superficial to the deep dermis with a top-heavy distribution, and a Grenz zone. (H and E, 2x). (b) Reactive germinal centre formation (H and E, 10x) with (c) small and mature lymphocytes, without atypia. (H and E,40x)
Figure 3.
Immunohistochemistry reveals a polymorphous infiltrate with a mixture of B and T cells, highlighted by CD20 (a) and CD3 (b) stains. Germinal centres have a high proliferative index (c), highlighted by Ki67
CPL is a benign reactive lymphoid proliferation in the skin that clinically and histologically simulates cutaneous lymphoma. Its aetiopathogenesis seems attributable to an exaggerated local immunologic reaction to different stimuli, such as drugs, vaccines, tattoos, arthropod bites or infections.[2] Vaccination site-associated pseudolymphoma is a rare phenomenon, reported with some vaccines, such as hepatitis A, hepatitis B, quadrivalent human papillomavirus, influenza, meningoencephalitis and tetanus.[2,3] It has been suggested that these lesions may be a delayed hypersensitivity reaction to aluminium hydroxide, a vaccine adjuvant used to increase its efficacy and promote immunogenicity.[2,3] The possibility of developing a second pseudolymphoma after a new injection of the same vaccine has also been reported.[3]
Two long registry-based studies about cutaneous reactions after SARS-CoV-2 vaccination have been published, without reporting any CPL cases among them.[1] Another case of CPL associated with the SARS-CoV-2 mRNA vaccine has been described,[4] presenting with similar characteristics to our case. Interestingly, unlike previously published cases of pseudolymphomas associated with other vaccines, neither of the two cases showed granulomatous pattern or necrosis in the histological examination. A possible explanation to different histological appearances of these two cases is that they are not related to aluminium hydroxide as this vaccine does not contain it.[5] More recently, a case of CPL has been described appearing on the blue ink of a tattoo shortly following a COVID-19 vaccination, suggesting COVID-19 vaccination may also worsen or precipitate the development of cutaneous inflammatory disorders, such as in tattoos that were previously well-tolerated.[6] Other cases of primary cutaneous lymphoproliferative disorders (PCLDs) developed following COVID-19 vaccination have been reported in the literature and are summarized in Table 1.[1,4,7,8,9,10,11,12]
Table 1.
Primary cutaneous lymphoproliferative disorders (PCLDs) developed following COVID-19 vaccination reported in the literature
| Study | Month, year | Country | Diagnosis | n | Age (years) and sex (M/F) | Vaccine |
|---|---|---|---|---|---|---|
| New onset condition | ||||||
| Català A | January, 2022 | Spain | Cutaneous B lymphoma | 1 | Unknown | Unknown |
| Bresler SC | November, 2022 | USA | Primary cutaneous CD8+ peripheral T-cell lymphoma | 1 | 62 F | Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | CD4+ PCSMLPD | 2 | 52 F 45 M |
Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | Sézary syndrome | 1 | 80 F | Pfizer-BioNTech |
| Hooper MJ | July, 2022 | USA | LyP type A | 2 | 50 M 20 F |
Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | LyP type A | 2 | 60 M 61 F |
Pfize-BioNTech |
| Koumaki D | July, 2022 | Greece | LyP type D | 2 | 60 F 66 F |
1 AstraZeneca 1 Pfizer-BioNTech |
| Bresler SC | November, 2022 | USA | Lymphomatoid reaction vs. LyP | 1 | 53 M | Moderna |
| Avallone G | November, 2022 | Italy | Atypical PLEVA | 1 | 62 F | AstraZeneca and Pfizer-BioNTech |
| Hooper MJ | July, 2022 | USA | PLEVA | 2 | 50 F “late teens” M | Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | CPL | 2 | 55 M 55 M |
Pfizer-BioNTech |
| Mintoff D | January, 2022 | Malta | CPL | 1 | 68 F | Pfizer-BioNTech |
| Hooper MJ | July, 2022 | USA | CPL | 2 | 70 F 50 M |
Pfizer-BioNTech |
| Verdaguer J | 2023 | Spain | CPL | 1 | 51 F | Pfizer-BioNTech |
| Recurrence/reactivation of latent pre-existing disease | ||||||
| Brumfiel CM | April, 2021 | USA | Primary cutaneous anaplastic large-cell lymphoma | 1 | 79 M | Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | CD4+PCSMLPD | 1 | 49 M | Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | Sézary syndrome | 2 | 58 M 61 M |
Pfizer-BioNTech |
| Panou E | February, 2022 | Greece | Large-cell transformation of a folliculotropic MF | 1 | 60 M | AstraZeneca |
| Avallone G | November, 2022 | Italy | Erythrodermic MF | 1 | 61 M | Pfizer-BioNTech |
| Panou E | February, 2022 | Greece | LyP type A | 1 | 73 F | AstraZeneca |
| Avallone G | November, 2022 | Italy | LyP type A | 1 | 67 M | Pfizer-BioNTech |
| Avallone G | November, 2022 | Italy | CPL | 1 | 47 F | Pfizer-BioNTech |
LyP: Lymphomatoid papulosis; PLEVA: Pityriasis lichenoides et varioliformis acuta; CD4+ PCSMLPD: Primary cutaneous CD4+small/medium T-cell lymphoproliferative disorder; CPL: Cutaneous pseudolymphoma; MF: Mycosis fungoides
This article presents another case of CPL associated with the SARS-CoV-2 vaccine. Unlike previous cases of vaccine-associated pseudolymphomas, it presents with some subtle different histological features and no involvement of aluminium in the pathogenic mechanism. Although rare, clinicians should be aware of this phenomenon, as with the rapid generalization of SARS-CoV-2 vaccine worldwide, new cases of vaccine-associated CPL are expected.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors sincerely thank the patient for her willingness to the publication of this case report.
References
- 1.Català A, Muñoz-Santos C, Galván-Casas C, Roncero Riesco M, Revilla Nebreda D, Solá-Truyols A, et al. Cutaneous reactions after SARS-CoV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142–52. doi: 10.1111/bjd.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos Pinheiro R, Duarte B, João A, Lencastre A. Cutaneous pseudolymphoma following quadrivalent human papillomavirus vaccination-a rare adverse event. J Dtsch Dermatol Ges. 2018;16:465–7. doi: 10.1111/ddg.13470. [DOI] [PubMed] [Google Scholar]
- 3.Cerroni L, Borroni RG, Massone C, Chott A, Kerl H. Cutaneous B-cell Pseudolymphoma at the Site of Vaccination. Am J Dermatopathol. 2007;29:538–42. doi: 10.1097/DAD.0b013e3181591bea. [DOI] [PubMed] [Google Scholar]
- 4.Mintoff D, Scerri L, Betts A. SARS-CoV-2 mRNA vaccine injection site pseudolymphoma. J Eur Acad Dermatol Venereol. 2022;36:e20–2. doi: 10.1111/jdv.17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfizer, Inc. Fact sheet for recipients and caregivers about Pfizer-BioNTech COVID-19 vaccine, bivalent which has emergency use authorization (EUA) to prevent coronavirus disease 2019 (COVID-19) 2023. [Last accessed on 2023 Jun 30]. pp. 1–9. Available from:https://www.fda.gov/media/167212/download .
- 6.Ramesh M, Hope L, Cockerell C, Hope R. Late onset pseudolymphomatous reaction to blue tattoo pigment precipitated by covid vaccination. SKIN J Cutan Med. 2023;7:594–7. [Google Scholar]
- 7.Bresler SC, Menge TD, Tejasvi T, Carty SA, Hristov AC. Two cases of challenging cutaneous lymphoid infiltrates presenting in the context of COVID-19 vaccination: A reactive lymphomatoid papulosis-like eruption and a bona fide lymphoma. J Cutan Pathol. 2023;50:213–9. doi: 10.1111/cup.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avallone G, Maronese CA, Conforti C, Fava P, Gargiulo L, Marzano AV, et al. Real-world data on primary cutaneous lymphoproliferative disorders following SARS-CoV-2 vaccination: A multicentre experience from tertiary referral hospitals. J Eur Acad Dermatol Venereol. 2023;37:e451–5. doi: 10.1111/jdv.18806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper MJ, Veon FL, LeWitt TM, Chung C, Choi J, Zhou XA, et al. Cutaneous T-Cell–rich lymphoid infiltrates after SARS-CoV-2 vaccination. JAMA Dermatol. 2022;158:1073–6. doi: 10.1001/jamadermatol.2022.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koumaki D, Marinos L, Nikolaou V, Papadakis M, Zografaki K, Lagoudaki E, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900–2. doi: 10.1111/ijd.16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brumfiel CM, Patel MH, DiCaudo DJ, Rosenthal AC, Pittelkow MR, Mangold AR. Recurrence of primary cutaneous CD30-positive lymphoproliferative disorder following COVID-19 vaccination. Leuk Lymphoma. 2021;62:2554–5. doi: 10.1080/10428194.2021.1924371. [DOI] [PubMed] [Google Scholar]
- 12.Panou E, Nikolaou V, Marinos L, Kallambou S, Sidiropoulou P, Gerochristou M, et al. Recurrence of cutaneous T-cell lymphoma post viral vector COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2022;36:e91–3. doi: 10.1111/jdv.17736. [DOI] [PMC free article] [PubMed] [Google Scholar]