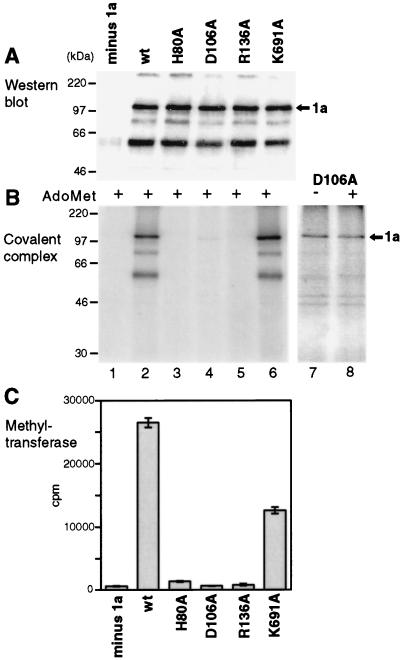
FIG. 7.
Effects of point mutations on enzymatic activities of 1a. Membrane fractions from yeast lacking 1a (lane 1), expressing wt 1a (lane 2), or expressing the indicated point mutant derivatives of 1a (lanes 3 to 6) were analyzed by Western blotting with 1a antiserum (A). The same fractions were assayed for covalent m7GMP complex formation in the presence of AdoMet (B) and for guanine-7-methyltransferase activity (C). The histogram bars of panel C show averages and standard deviations from assays of two complete sets of such fractions, each in duplicate. A longer exposure of the covalent guanylate binding activity of mutant D106A is shown in panel B, lanes 7 and 8, assayed in the absence or presence of AdoMet, as indicated. Arrows mark the position of 1a, and positions of molecular weight markers are shown at the left.