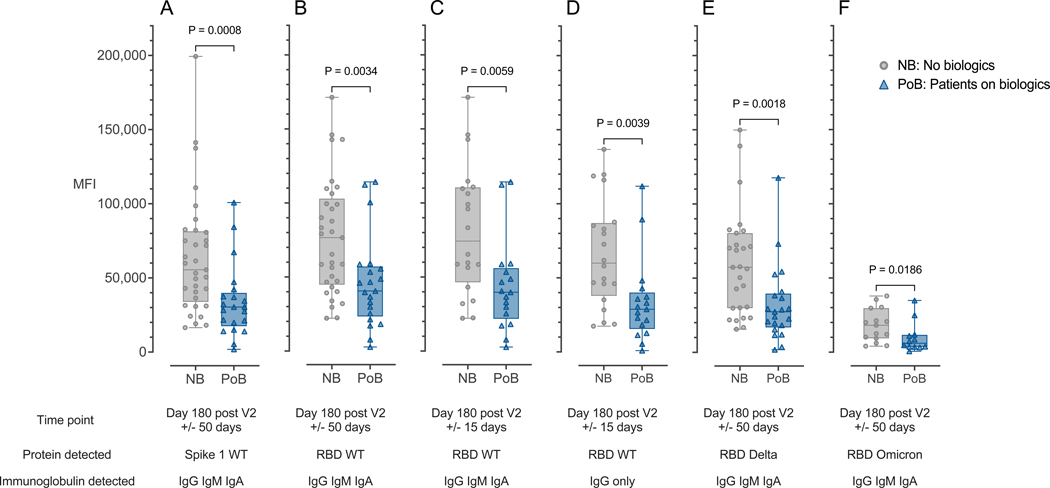
Figure 2. Antibody binding is correlated with antibody levels.
(A) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 Spike 1 protein measured at day 180 after V2 +/− 50 days. (B) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. (C) Combined IgA, IgG, and IgM immunoglobulins binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 15 days. (D) Immunoglobulin IgG only binding to WT SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 15 days. (E) Combined IgA, IgG, and IgM immunoglobulins binding to the Delta-variant SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. (F) Combined IgA, IgG, and IgM immunoglobulins binding to the Omicron-variant SARS-CoV-2 RBD protein measured at day 180 after V2 +/− 50 days. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab or mepolizumab; IgA, Immunoglobulin A; IgM, Immunoglobulin M; IgG, Immunoglobulin G; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; RBD, receptor-binding domain; MFI, median fluorescence intensity; V2, second mRNA SARS-CoV-2 vaccination dose.