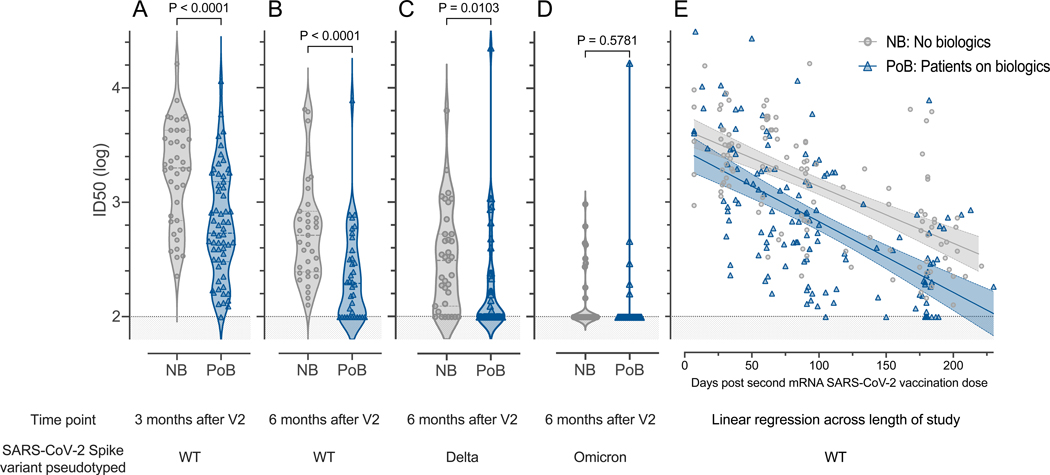
Figure 3. Pseudovirus neutralization.
All comparisons were performed between subjects not on biologics (NB) and patients on biologics (PoB) (A) Pseudovirus neutralization of WT SARS-CoV-2, comparison between three months after V2. (B) Pseudovirus neutralization of WT SARS-CoV-2 six months after V2. (C) Pseudovirus neutralization of Delta-variant SARS-CoV-2 six months after V2. (D) Pseudovirus neutralization of Omicron-variant BA.1 SARS-CoV-2 six months after V2. (E) Linear regression of data points from each group (NB and PoB) across the entire study follow-up period. Their 95% confidence intervals are represented by the shaded area. For all subfigures: the shaded area under 2 represents the lower limit of detection of the assay. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab and mepolizumab; V2, dose two of the SARS-CoV-2 mRNA vaccine; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution.