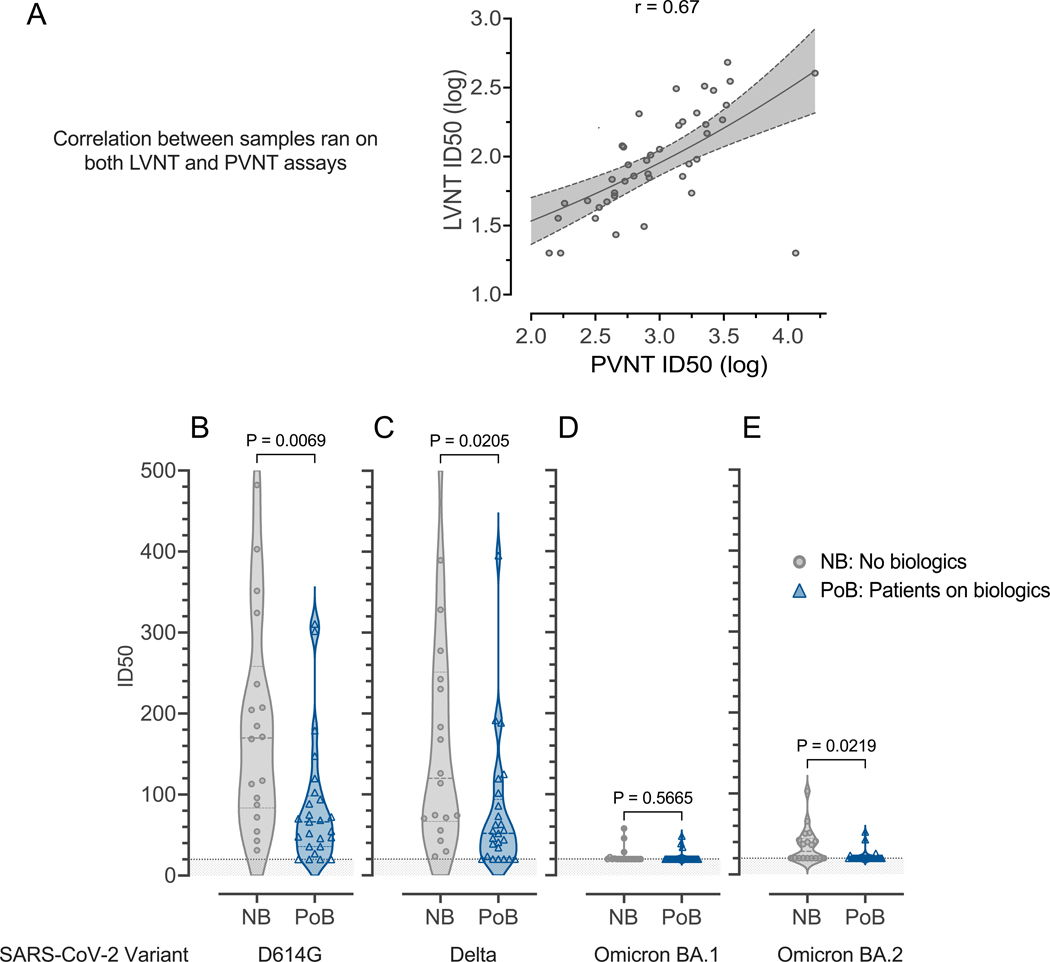
Figure 4. Live virus neutralization.
Samples collected between days 69 and 105 after the second mRNA SARS-CoV-2 vaccination (A) Correlation between live-virus neutralization and pseudovirus neutralization for samples run on both assays. (B) Live-virus neutralization of D614G-variant SARS-CoV-2. (C) Live-virus neutralization of Delta-variant SARS-CoV-2. (D) Live virus neutralization of Omicron-variant BA.1 SARS-CoV-2. (E) Live virus neutralization of Omicron-variant BA.2 SARS-CoV-2. For all subfigures: the shaded area under 20 represents the lower limit of detection of the LVNT assay. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab, and mepolizumab; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild-type; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution; PVNT, pseudovirus neutralization; LVNT, live virus neutralization.