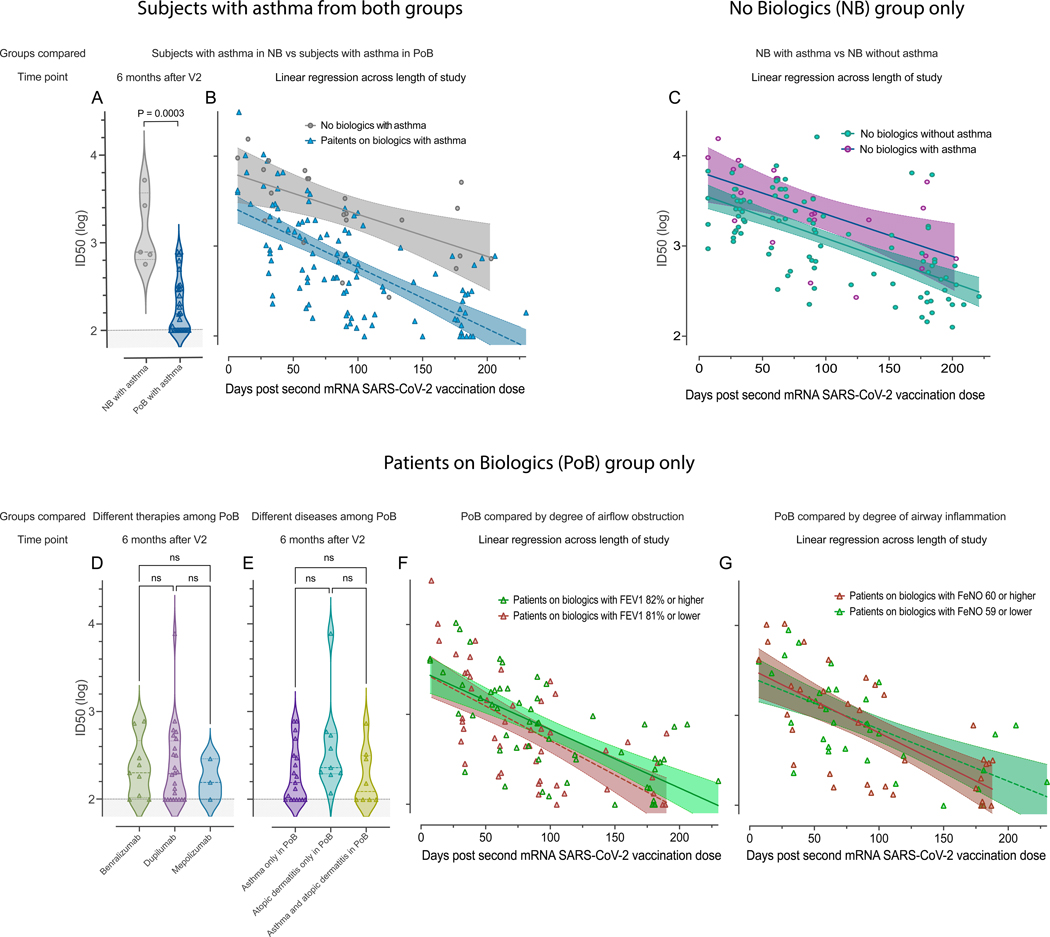
Figure 5. Subgroup analyses.
Pseudovirus neutralization of WT SARS-CoV-2, comparison between subjects with asthma in the NB group and subjects with asthma in the PoB group (A) six months after V2 and (B) across the entire study follow-up period. (C) Pseudovirus neutralization of WT SARS-CoV-2, comparison between subjects with and without asthma in the no biologics control group across the entire study follow-up period. (D) Pseudovirus neutralization of WT SARS-CoV-2, comparison between different therapies (benralizumab, dupilumab, or mepolizumab) among all PoB six months after V2. (E) Pseudovirus neutralization of WT SARS-CoV-2, comparison between diseases (asthma, atopic dermatitis, or both) among all PoB six months after V2. (F) Pseudovirus neutralization of WT SARS-CoV-2 of patients with asthma on biologics stratified by the degree of airway obstruction across the entire study follow-up period. In green: patients with asthma on biologics who have an FEV1 of 82% of predicted or higher. In red: patients with asthma on biologics who have an FEV1 of 81% of predicted or lower. (G) Pseudovirus neutralization of WT SARS-CoV-2 of patients with asthma on biologics stratified by degree of airway inflammation across the entire study follow-up period. In red: patient with asthma on biologics who have a FeNO of 60ppb or higher. In green: patients with asthma on biologics who have a FeNO of 59 or lower. For subfigures A, D, and E: the shaded area under 2 represents the lower limit of detection of the assay. For subfigures B, C, F, and G: the diagonal lines represent the linear regression of data points from each group and their 95% confidence intervals are represented by the shaded area. NB, control subjects not on biologics; PoB, patients on biologics include all subjects treated with benralizumab, dupilumab and mepolizumab; V2, dose two of the SARS-CoV-2 mRNA vaccine; WT, wild-type; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger ribonucleic acid; ID50, 50% inhibitory dilution; ns, non-significant p>0.05; FEV1, forced expiratory volume in the first second; FeNO, fractional exhaled nitric oxide.