Abstract
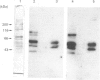
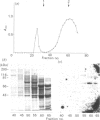
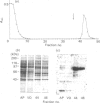
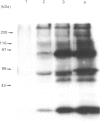
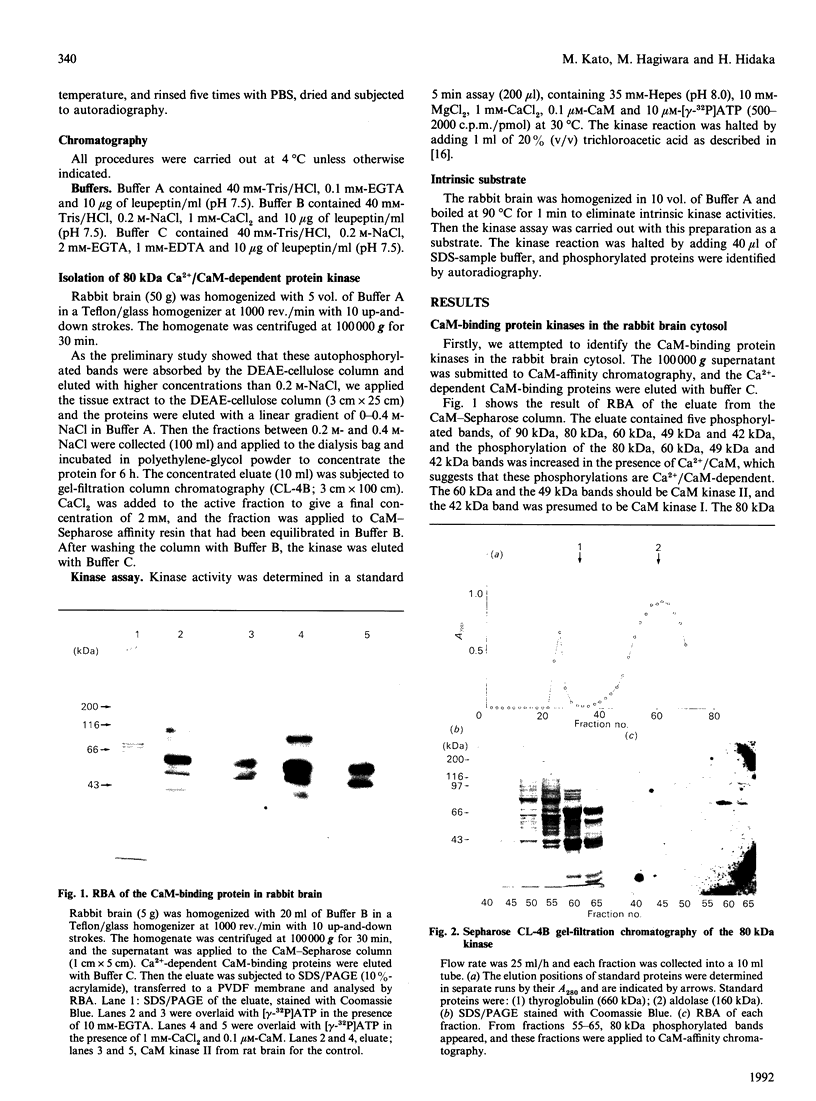
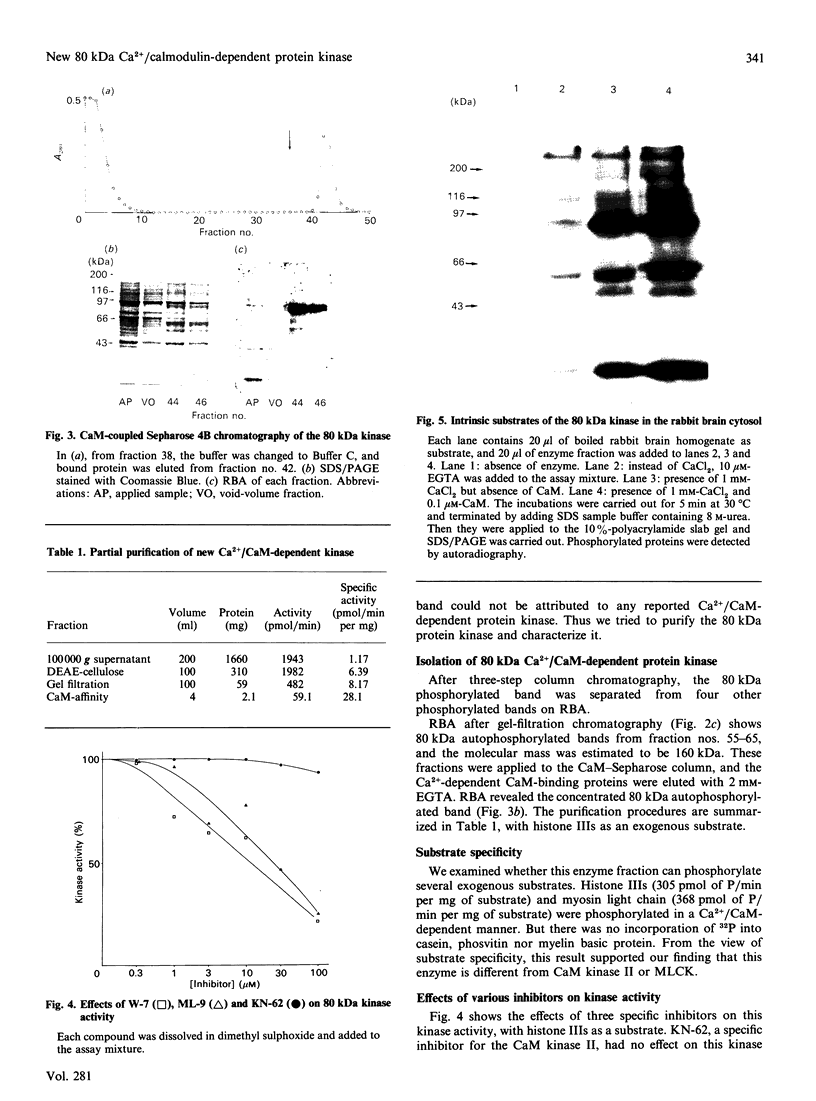
We surveyed rabbit brain cytosol for a new Ca2+/calmodulin (CaM)-dependent kinase. The renaturation blotting assay (RBA) exploits the ability of blotted SDS-denatured proteins to regain enzymic activity after guanidine treatment. Using RBA, we found that the eluate of rabbit brain cytosol from a CaM affinity column contains at least four electrophoretically distinct protein kinase bands which were autophosphorylated in a Ca2+/CaM-dependent manner. The 49 kDa band and the 60 kDa band were alpha and beta subunit of CaM kinase II, and the 42 kDa band was presumed to be CaM kinase I, but the 80 kDa band could not be attributed to any reported Ca2+/CaM-dependent protein kinases. The 80 kDa protein kinase was isolated by three-step chromatography. We examined the phosphorylation of exogenous substrates by 80 kDa protein kinase, and histone IIIs and myosin light chain were phosphorylated in a Ca2+/CaM-dependent manner. W-7, a specific inhibitor for calmodulin, inhibited this kinase activity, but KN-62, a specific inhibitor for CaM kinase II, had no effect on this protein kinase activity. Autoradiography using boiled rabbit brain homogenate as substrate showed three intrinsic substrates (80 kDa, 60 kDa and 42 kDa), which were phosphorylated in a Ca2+/CaM-dependent manner. These findings suggest that a new Ca2+/CaM-dependent protein kinase could be identified by the RBA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Schworer C. M., Hashimoto Y., Fong Y. L., Rich D. P., Smith M. K., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Biochem J. 1989 Mar 1;258(2):313–325. doi: 10.1042/bj2580313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Tanaka T., Isobe T., Kasai H., Okuyama T., Hidaka H. Calcium-dependent affinity chromatography of S-100 and calmodulin on calmodulin antagonist-coupled Sepharose. J Biol Chem. 1981 Dec 10;256(23):12485–12489. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Hagiwara M., Inagaki M., Watanabe M., Ito M., Onoda K., Tanaka T., Hidaka H. Selective modulation of calcium-dependent myosin phosphorylation by novel protein kinase inhibitors, isoquinolinesulfonamide derivatives. Mol Pharmacol. 1987 Jul;32(1):7–12. [PubMed] [Google Scholar]
- Hagiwara M., Tokumitsu H., Onoda K., Tanaka T., Ito M., Kato N., Hidaka H. Monoclonal antibody assessment of tissue- and species-specific myosin light chain kinase isozymes. J Biochem. 1989 Jul;106(1):71–75. doi: 10.1093/oxfordjournals.jbchem.a122822. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- Miyamoto E., Kakiuchi S. In vitro and in vivo phosphorylation of myelin basic protein by exogenous and endogenous adenosine 3':5'-monophosphate-dependent protein kinases in brain. J Biol Chem. 1974 May 10;249(9):2769–2777. [PubMed] [Google Scholar]
- Nairn A. C., Bhagat B., Palfrey H. C. Identification of calmodulin-dependent protein kinase III and its major Mr 100,000 substrate in mammalian tissues. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7939–7943. doi: 10.1073/pnas.82.23.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M., Ishikawa T., Matsushima S., Naka M., Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987 Jun 5;262(16):7796–7801. [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990 Mar 15;265(8):4315–4320. [PubMed] [Google Scholar]
- Wakim B. T., Picken M. M., DeLange R. J. Identification and partial purification of a chromatin bound calmodulin activated histone 3 kinase from calf thymus. Biochem Biophys Res Commun. 1990 Aug 31;171(1):84–90. doi: 10.1016/0006-291x(90)91359-z. [DOI] [PubMed] [Google Scholar]