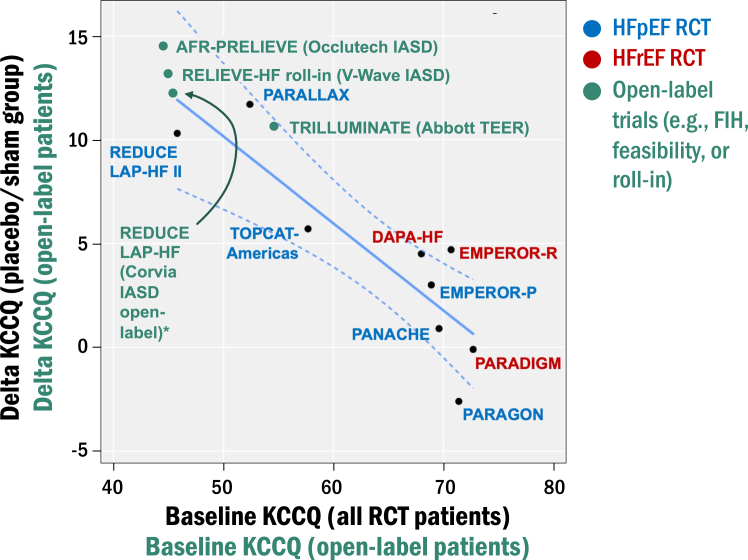
Figure 2.
Effect of placebo/sham on health status (Kansas City Cardiomyopathy Questionnaire [KCCQ] scores) in completed heart failure (HF) randomized clinical trial (RCT) compared with open-label interventional HF trials. For the pharmacological HF trials, baseline KCCQ scores correlated inversely with delta KCCQ scores in the placebo group (R2 = 0.76, P = .002), suggesting that the change in KCCQ scores over time is dependent on the starting (baseline) KCCQ score. This is particularly relevant to open-label interventional HF trials because baseline KCCQ scores tend to be lower than most pharmacological HF trials except those such as PARALLAX, which have an upper threshold for KCCQ values. In the RCT, patients in the placebo group are blinded, whereas patients in the open-label interventional HF trials are unblinded, which may enhance placebo effects, as suggested by the higher delta KCCQ in the unblinded (open-label) vs blinded (sham control) patients in the Corvia REDUCE LAP-HF vs REDUCE LAP-HF II trials, respectively. FIH, first-in-human.