Abstract
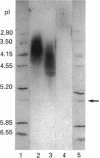
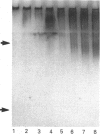
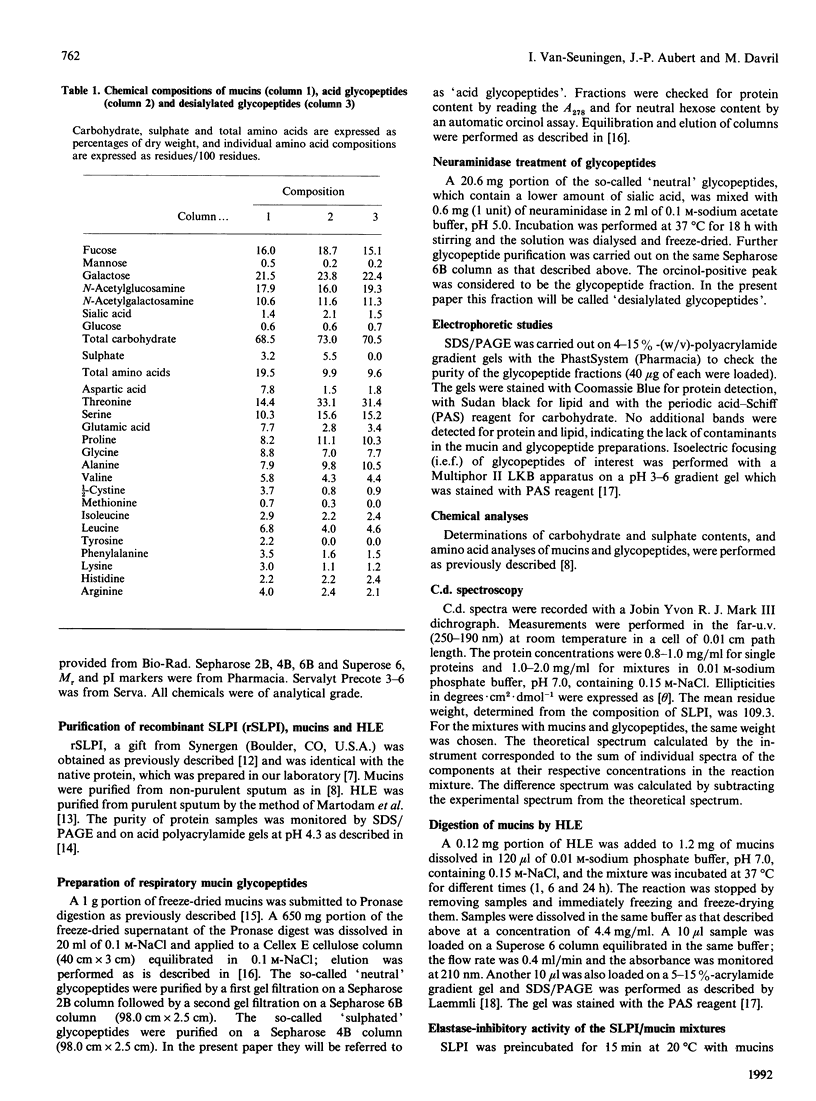
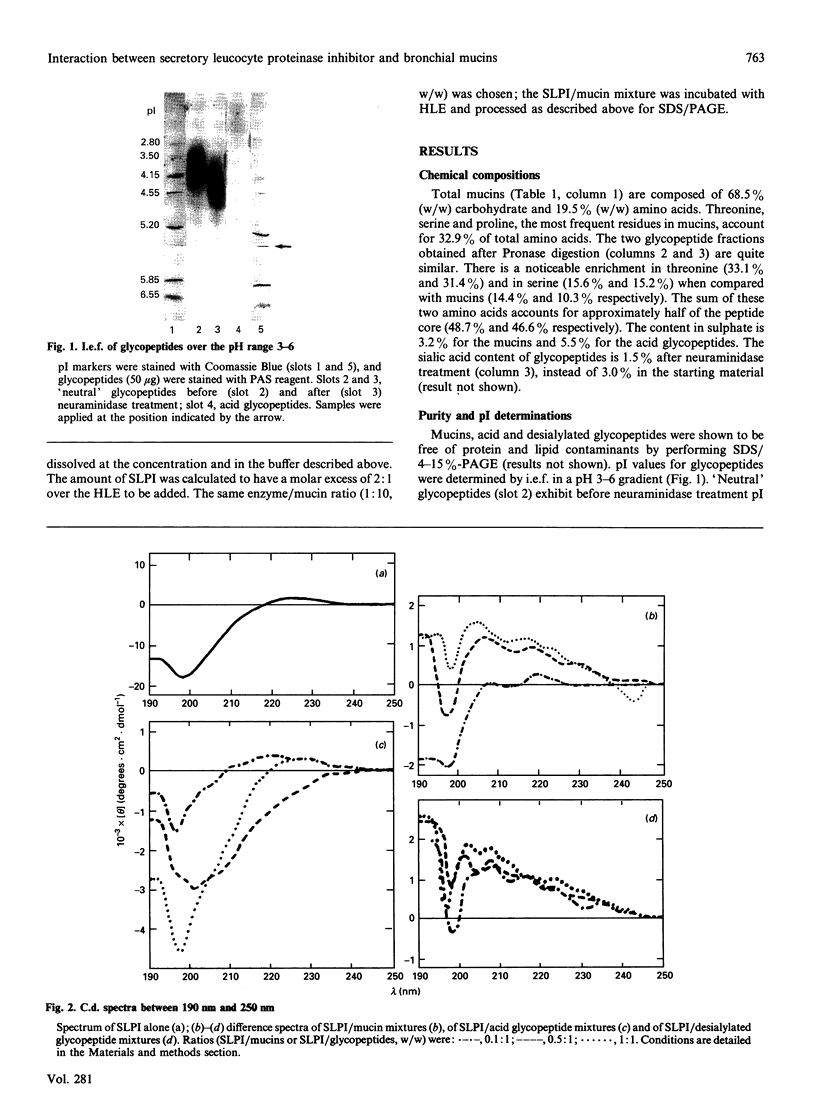
The interaction of secretory leucocyte proteinase inhibitor with bronchial mucins and glycopeptides was studied by means of c.d. spectroscopy. The interaction with mucins was characterized by an increase in organized structure of alpha-helical type, as evidenced by the appearance in the difference spectra of two positive bands at 208 and 218 nm. This phenomenon was correlated with the amount of inhibitor present in the mixtures, suggesting that the change was inherent to the inhibitor. Surprisingly, when the inhibitor was mixed with acid glycopeptides, difference c.d. spectra showed a decrease in organized structure, characterized by a negative minimum at 196 nm. Glycopeptides treated with neuraminidase gave similar profiles of difference spectra in three different mixtures, indicating that the interaction was smaller. The interaction between the inhibitor and mucins was also studied for its ability to modify in vitro the proteolytic activity of human leucocyte elastase. Mucins alone were degraded by that proteinase into glycopeptides of Mr 400,000-500,000, whereas mucins mixed with inhibitor before adding elastase were proteolysed to a lesser extent. These data demonstrate that the secretory leucocyte proteinase inhibitor interacts with mucins and consequently is capable of protecting the mucins against proteolysis by elastase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breuer R., Christensen T. G., Lucey E. C., Stone P. J., Snider G. L. Quantitative study of secretory cell metaplasia induced by human neutrophil elastase in the large bronchi of hamsters. J Lab Clin Med. 1985 May;105(5):635–640. [PubMed] [Google Scholar]
- Cheng P. W., Boat T. F., Cranfill K., Yankaskas J. R., Boucher R. C. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Invest. 1989 Jul;84(1):68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Bridge J. L., Horton J. R. An interaction between lysozyme and mucus glycoproteins. Implications for density-gradient separations. Biochem J. 1979 Sep 1;181(3):717–724. doi: 10.1042/bj1810717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Hale K. K., Heimdal P., Thompson R. C. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem. 1990 May 15;265(14):7976–7981. [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Franken C., Meijer C. J., Dijkman J. H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989 Apr;37(4):493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbitz O., Jenssen A. O., Smidsrød O. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. Eur J Respir Dis. 1984 Oct;65(7):512–520. [PubMed] [Google Scholar]
- Kim K. C., Wasano K., Niles R. M., Schuster J. E., Stone P. J., Brody J. S. Human neutrophil elastase releases cell surface mucins from primary cultures of hamster tracheal epithelial cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9304–9308. doi: 10.1073/pnas.84.24.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Lamblin G., Lhermitte M., Roussel P., Breg J., Van Halbeek H., Vliegenthart J. F. Primary structure of neutral oligosaccharides derived from respiratory-mucus glycoproteins of a patient suffering from bronchiectasis, determined by combination of 500-MHz 1H-NMR spectroscopy and quantitative sugar analysis. 1. Structure of 16 oligosaccharides having the Gal beta(1----3)GalNAc-ol core (type 1) or the Gal beta(1----3)[GlcNAc beta(1----6)]GalNac-ol core (type 2). Eur J Biochem. 1988 Feb 1;171(3):631–642. doi: 10.1111/j.1432-1033.1988.tb13834.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamblin G., Lhermitte M., Degand P., Roussel P., Slayter H. S. Chemical and physical properties of human bronchial mucus glycoproteins. Biochimie. 1979;61(1):23–43. doi: 10.1016/s0300-9084(79)80310-7. [DOI] [PubMed] [Google Scholar]
- Martodam R. R., Baugh R. J., Twumasi D. Y., Liener I. E. A rapid procedure for the large scale purification of elastase and cathepsin G from human sputum. Prep Biochem. 1979;9(1):15–31. doi: 10.1080/00327487908061669. [DOI] [PubMed] [Google Scholar]
- Poncz L., Jentoft N., Ho M. C., Dearborn D. G. Kinetics of proteolysis of hog gastric mucin by human neutrophil elastase and by Pseudomonas aeruginosa elastase. Infect Immun. 1988 Mar;56(3):703–704. doi: 10.1128/iai.56.3.703-704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Lhermitte M., Houdret N., Lafitte J. J., Perini J. M., Klein A., Scharfman A. The complexity of mucins. Biochimie. 1988 Nov;70(11):1471–1482. doi: 10.1016/0300-9084(88)90284-2. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Lucey E. C., Christensen T. G., Stone P. J., Calore J. D., Catanese A., Franzblau C. Emphysema and bronchial secretory cell metaplasia induced in hamsters by human neutrophil products. Am Rev Respir Dis. 1984 Jan;129(1):155–160. doi: 10.1164/arrd.1984.129.1.155. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van-Seuningen I., Davril M. Electrotransfer of basic proteins from nondenaturing polyacrylamide acid gels to nitrocellulose: detection of enzymatic and inhibitory activities and retention of protein antigenicity. Anal Biochem. 1990 May 1;186(2):306–311. doi: 10.1016/0003-2697(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Van-Seuningen I., Davril M., Hayem A. Evidence for the tight binding of human mucus proteinase inhibitor to highly glycosylated macromolecules in sputum. Biol Chem Hoppe Seyler. 1989 Jul;370(7):749–755. doi: 10.1515/bchm3.1989.370.2.749. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Hubbard R. C., Fells G. A., Schnebli H. P., Thompson R. C., Fritz H., Crystal R. G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991 Feb;87(2):482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]