Abstract
Background and aims
With existing literature focusing on general quality of life, the magnitude and impact of depression among recipients after liver transplantation (LT) is unclear. Hence, we aim to evaluate the prevalence, risk factors, and outcomes for recipient-related depression after LT.
Methods
Medline and Embase were searched. Single-arm analysis was pooled using the generalized linear mixed model, and logistic regression was performed to analyze risk factors. Pairwise comparative meta-analysis in odds ratio was conducted for binary outcomes.
Results
Of 1069 abstracts, 189 articles underwent full-text review before the inclusion of 48 articles. Pooled depression rate among 5170 recipients was 24.52% (confidence interval [CI]: 19.46%–30.41%). Depression was most prevalent in Asia compared with other geographical regions. Younger age at transplantation (P = .019) and university education (P = .051) were protective against depression. However, those transplanted for alcoholic liver disease (odds ratio: 1.14, CI: 1.10–1.18, P ≤ 0.001) were more likely to be depressed. Depression resulted in increased odds of mortality (odds ratio: 1.82, CI: 1.08–3.07, P = .04), graft loss (P = .03), and graft rejection (P = .01).
Conclusion
Depression is highly prevalent after LT and may be associated with increased mortality and poorer graft outcomes. More emphasis is needed on the screening of depression among higher risk recipients.
Keywords: Psychological Wellbeing, Mood Disorders, Liver Transplantation, Mental Health
Introduction
Liver transplantation (LT) is the definitive treatment in patients with end-stage liver disease. With significant improvements in graft and patient survival over the past few decades, the focus on outcome measures has shifted toward inclusion of patient-reported quality of life (QOL). However, psychosocial challenges are highly prevalent after LT,1 with depression being identified in up to 40% of LT recipients.2 Although numerous studies have reported symptoms of depression in LT recipients, prevalence rates vary greatly, ranging from 17% to 40%.3,4 This variation may likely reflect differences in the psychometric tools used to assess these disorders, study population, and etiology of liver disease requiring LT among other factors. Clinically, presence of depression in LT recipients has been associated with decreased compliance with long-term medications5 and poorer clinical outcomes,6,7 including reduced survival when compared with their LT recipients without depression.2 In addition, LT recipients with depression reported greater difficulty in returning to work7 and lower levels of satisfaction with their lives.5
At present, the impact of LT on QOL has been well studied,1,8 but there remains a gap in literature on the pooled prevalence estimates and variables affecting the development of mood disorders in the LT population. Quantitative synthesis of depression in LT recipients is important in better understanding the mental health burden in this population, thereby allowing optimization of health services and support programs. Given the significant impact of depression on both psychological and physical wellbeing, identification of potential risk factors is critical to target health resources to the group that will benefit from it most. This meta-analysis, therefore, sought to evaluate the prevalence, risk factors, and outcomes of recipient-related depression after LT.
Methods
Search Strategy
A systematic literature search was conducted on Medline and Embase database for articles relating to any depression diagnosis in liver transplant recipients from inception to January 21, 2021. This review was registered in advance with PROSPERO (CRD42021231807).9 The search strategy used included MeSH and text word searches on “Liver Transplant” and “Depression”. No date restriction was applied. Identified abstracts were compiled, and duplicates were removed with EndNote X9 Software (Clarivate Analytics). In addition, the screening of references of relevant articles and a previous meta-analysis2 was also conducted to identify further eligible studies not covered by the original database searches. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines were adhered to in the synthesis of the review.10
Selection Criteria and Extraction
Prospective and retrospective cohort and cross-sectional studies assessing depression rates among LT recipients were included in the study. There were no restrictions in terms of gender, age, race, or ethnicity. Only original studies and English language articles were considered for inclusion, excluding reviews, commentaries, editorials, conference abstracts, and articles originating from the same database. Pediatric studies defined as those with a study population of 18 years of age or younger at the time of transplant were included. Other exclusion criteria included (1) studies that failed to provide sufficient information in the type of screening tools for depression, and (2) studies that did not provide sufficient data to calculate point prevalence of depression. The inclusion of an article was evaluated by 3 independent blinded authors (W.H.L., D.J.H.T., C.W.P.), with any disagreements being resolved by obtaining the consensus of a fourth author (M.D.M.). As with a previous review,11 identification of depression can be classified into (1) clinician-rated diagnosis based on diagnostic criteria as ascertained by clinical interviews or rating instruments administered by clinicians or researchers, (2) self-rated questionnaire based on rating instruments self-administered by participants, or (3) self-reported depression. A clinician-rated diagnosis includes psychiatric evaluation, diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision, or Hamilton Depression Rating Scale (HAMD). The self-rated questionnaire includes the use of validated depression scales (eg, BDI, Beck Depression Inventory; HADS, Hospital Anxiety and Depression Scale; patient health questionnaire; Children’s Depression Inventory; Korean Center for Epidemiological Studies-Depression Scale; EuroQOL-5 Dimension; SDS, Zung self-rating depression scale) while self-reported depression refers to patients’ self-identification of depressive symptoms after donation.
Outcomes and Definitions
Relevant data from each article including background information (eg, author, year, hospital, country, study design), baseline characteristics (eg, sample size, age, ethnicity, gender, and so on), the method of diagnosis, and prevalence of post-LT depression were extracted by 2 independent authors (C.W.P. and B.J.M.T.) onto a structured proforma. The main outcomes of interest in this meta-analysis were depression rates in LT recipients. Risk factors analysis included the following parameters: age, gender, indication for LT (hepatitis B, hepatitis C, alcoholic liver disease [ALD], nonalcoholic steatohepatitis), ethnicity (Caucasian, Hispanic, African-American), education level (elementary, high school, college, or university), employment status (part-time or full-time employment vs unemployed), and marital status (stable relationship or divorced). Consistent with previous studies,12,13 patients were categorized into age groups of younger than 25 years, 26–50 years, and older than 50 years. Comparisons between depressed and nondepressed LT recipients were conducted to evaluate the impact of depression on the following clinical and patient outcomes: mortality, graft loss, graft rejection, and alcohol use after LT. Graft loss included both graft failure and retransplantation while graft rejection and alcohol use after LT were defined according to individual studies. Alcohol use after LT was assessed in patients transplanted for ALD. For all included studies, a diagnosis of depression preceded the post-LT outcome of interest.
Statistical Analysis
All analyses were performed in STATA (StataCorp 16.1) and R studio (Version 1.3.1093). A detailed statistical analysis can be found in Supplementary Material 1. In brief, the proportion of individuals with symptoms of depression in each study was combined to give an overall pooled prevalence of depression after LT. Random effects model was used in all analyses regardless of heterogeneity as recent evidence suggests that it provides more robust outcome measures than the alternative fixed effects models.14 Statistical heterogeneity was assessed via I2 and Cochran Q test values, where an I2 value of 0%–40% indicates low heterogeneity, while values of 30%–60%, 50%–90%, and 75%–100% were classified as moderate, substantial, and considerable heterogeneity, respectively.15 A Cochran Q test with a P value of ≤.10 was considered significant for heterogeneity. However, traditional tools measuring heterogeneity for single-arm meta-analysis have been found to be inaccurate16 with several single-arm analyses exceeding I2 >90%.17,18 Various subgroup analyses were conducted, including but not limited to study population (ie, adults or pediatric), different diagnosis methods11 (ie, clinician-diagnosed, self-reported, and self-rated diagnosis), and various screening tools used19 (ie, BDI, HADS, Diagnostic and Statistical Manual of Mental Disorders, 4th edition, patient health questionnaire, Children’s Depression Inventory, Korean Center for Epidemiological Studies-Depression Scale, EuroQOL, HAMD, SDS). The rate of depression was also stratified based on the mean follow-up time20 (ie, before or after 5 years of follow-up) and geographical region (ie, Asia, North America, South America, Middle East, and Europe). Risk factors were then identified through a generalized linear model regression to obtain the odds ratio (OR). Finally, a pairwise meta-analysis was performed using the Dersimonnian-Laird random effects model15,21 to obtain the OR and 95% CI between depressed vs nondepressed patients. Statistical significance was considered for outcomes with P value <.05. Quality assessment of included articles was performed with Hoy et al22 tool for prevalence study, which assesses the risk of bias based on sampling population, validity of data collection, and appropriate study instruments across 10 domains.
Results
Summary of Included Articles
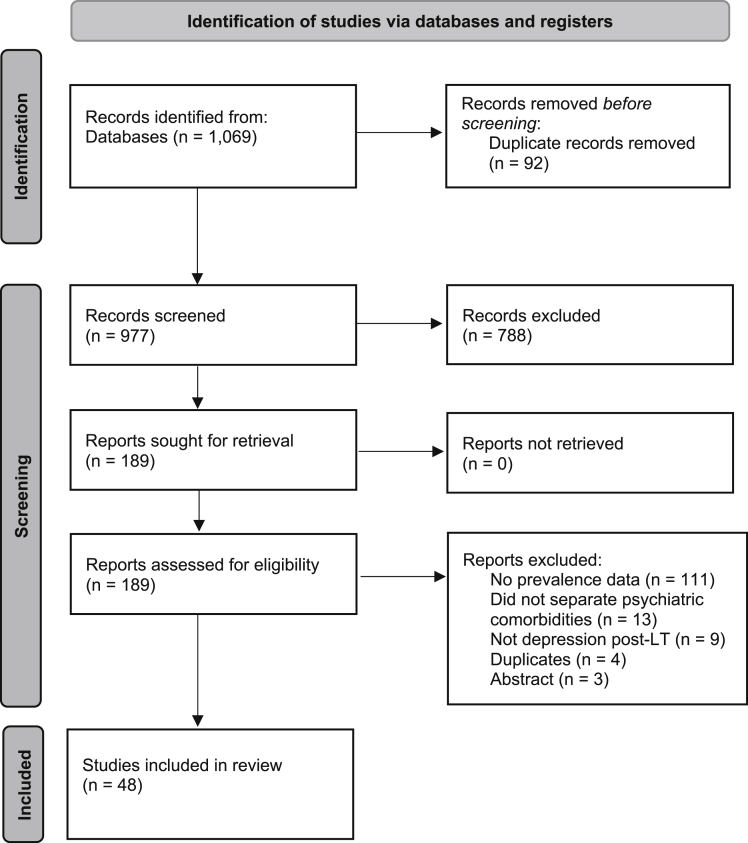
In total, 1069 articles were retrieved from the search after duplicates removal, with a final of 189 articles undergoing full-text review. Of the 189 articles, a final of 48 articles were included in this review (Figure 1). There were 9 longitudinal studies, while the majority were cross-sectional. There were 14 included studies from United States; 4 from United Kingdom; 3 each from Brazil, China, Italy, and Netherlands; 2 each from Egypt, Finland, Taiwan, Germany, Turkey, and Spain; and one each from Canada, Denmark, Iran, Poland, France, and Sweden. The articles included spanned from 1991 to 2020, with more articles using self-rated (n = 33) than clinician-rated, (n = 11) or self-reported depression (n = 4). In total, 5170 transplant recipients who underwent either living donor LT or deceased donor LT were assessed for depression after LT (median sample study size: 74.5). A total of 1478 individuals reported depression. Of the 48 articles, 42 articles involved adult LT recipients (mean age: 50.84 years), while 6 articles focused specifically on the pediatric LT population (mean age: 12.77 years). A total of 1431 out of 4817 adults (29.71%) were assessed to have depression, while 47 out of 353 children (13.31%) had depression. The mean follow-up duration for all LT recipients was 4.26 years. The summary of included articles and risk-of-bias assessment can be found in Supplementary Materials 2 and 3, respectively, with all included studies assessed to have low to moderate risk of bias.
Figure 1.
PRISMA flow diagram of included articles.
Prevalence of Depression
The pooled prevalence of depression among 5170 recipients after LT ranged from 3.74% (CI: 1.20%–9.85%) to 78.00% (CI: 63.67%–88.01%) with an overall meta-analytical prevalence of 24.52% (CI: 19.46%–30.41%). A summary of the pooled prevalence can be found in Table 1. Depression rates were 25.75% (CI: 20.29%–32.08%) and 16.64% (CI: 7.74%–32.20%) among 4817 adults and 353 children, respectively, without significant difference between groups (P = .10). Comparisons on depression point estimates across self-reported depression, self-rated instrument, and clinician-rated diagnoses yielded 52.80% (CI: 43.07%–62.32%, n = 604), 23.33% (CI: 17.84–29.89, n = 3782), and 19.38% (CI: 11.47–30.85, n = 784), respectively.
Table 1.
Pooled Prevalence of Depression
| Subgroups | No. of articles | Total sample size | Events | Pooled prevalence |
|---|---|---|---|---|
| Overall | 48 | 5170 | 1478 | 24.52% (CI: 19.46–30.41) |
| Adults only | 42 | 4817 | 1431 | 25.75% (CI: 20.29–32.08) |
| Paediatrics only | 6 | 353 | 47 | 16.64% (CI: 7.74–32.20) |
| Mean follow-up time | ||||
| 5 y or less | 22 | 2048 | 541 | 26.21% (CI: 19.51–34.75) |
| More than 5 y | 6 | 540 | 104 | 18.98% (CI: 12.95–26.94) |
| Diagnosis method | ||||
| Self-rated | 33 | 3782 | 1004 | 23.33% (CI: 17.84–29.89) |
| Clinician-rated | 11 | 784 | 173 | 19.38% (CI: 11.47–30.85) |
| Self-reported | 4 | 604 | 301 | 52.80% (CI: 43.07–62.32) |
| Screening tool | ||||
| BDI | 11 | 1268 | 314 | 21.24% (CI: 14.92–29.31) |
| HADS | 11 | 1150 | 226 | 18.73% (CI: 12.25–27.56) |
| DSM-IV | 5 | 325 | 64 | 18.22% (CI: 11.39–27.87) |
| PHQ-9 | 4 | 292 | 161 | 49.20% (CI: 21.08–77.84) |
| CDI | 2 | 158 | 17 | 14.59% (CI: 4.41–38.73) |
| CES-D | 2 | 432 | 91 | 22.03% (CI: 14.40–32.19) |
| EQ5D | 2 | 226 | 53 | 23.45% (CI: 18.38–29.42) |
| HAMD | 1 | 75 | 5 | 6.67% (CI: 2.80–15.04) |
| SDS | 1 | 256 | 142 | 55.47% (CI: 49.33–61.45) |
| Geographical region | ||||
| Asia | 5 | 801 | 292 | 32.09% (CI: 15.59–54.73) |
| North America | 15 | 1536 | 469 | 29.64% (CI: 19.38–42.48) |
| South America | 3 | 236 | 75 | 31.78% (CI: 26.16–37.99) |
| Middle East | 5 | 343 | 75 | 15.72% (CI: 5.56–37.12) |
| Europe | 20 | 2254 | 567 | 20.98% (CI: 15.24–28.15) |
BDI, Beck Depression Inventory; CDI, Children’s Depression Inventory; CES-D, Korean Center for Epidemiological Studies-Depression Scale; DSM IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; EQ5D, EuroQOL-5, Dimension; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Rating Scale; PHQ-9, patient health questionnaire; SDS, Zung self-rating depression scale.
A separate subgroup analysis was conducted based on the tool used to measure depression. Of the 49 studies reporting depression rates in LT recipients after transplant, 11 used BDI and 11 used HADS, with a pooled prevalence of 21.24% (CI: 14.92%–29.31%) and 18.73% (CI: 12.25%–27.56%), respectively. Among studies that used other questionnaires, the lowest depression rate was reported in one study that used HAMD (6.67%, CI: 2.80%–15.04%), compared with the highest depression rate in a study using SDS (55.47%, CI: 49.33%–61.45%).
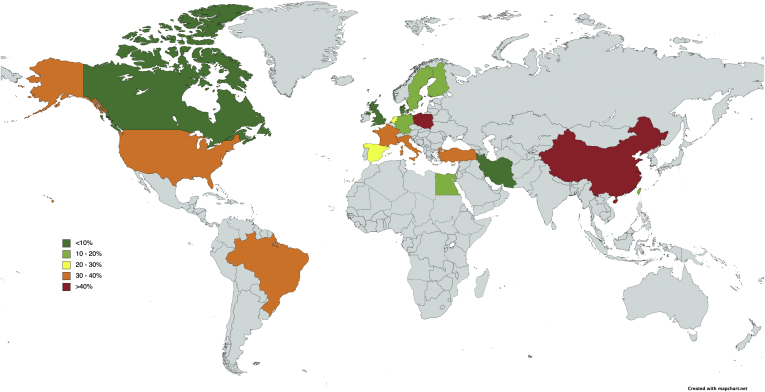
A subgroup analysis was performed to account for differences in depression rates based on geographical regions (Table 1 and Figure 2). The prevalence of depression was 32.09% (CI: 15.59%–54.73%, n = 5) in Asia, 31.78% (CI: 26.16%–37.99%, n = 3) in South America, 29.64% (CI: 19.38%–42.48%, n = 15) in North America, 20.98% (CI: 15.24%–28.15%, n = 20) in Europe, and 15.72% (CI: 5.56%–37.12%, n = 5) in Middle East. Depression rates were lower for studies with longer than 5 years of follow-up at 18.98% (CI: 12.95–26.94, n = 6) than those for studies with shorter than 5 years of follow-up at 26.21% (CI: 19.51–34.75, n = 22) although both groups were not significantly different (P = .24).
Figure 2.
Map of depression rates by country.
Risk Factors of Depression
The baseline characteristics of patients in the included studies were used as risk factor adjustment for depression, and the results are summarized in Table 2. University education was found to be a preventive factor against depression after LT (OR: 0.34, CI: 0.17–0.67, P = .051). In the analysis of indications for LT, patients who were transplanted for ALD were more likely to be depressed (OR: 1.14, CI: 1.10–1.18, P ≤ 0.001). By and large, age, ethnicity, gender, employment, and marital status did not affect depression rates among LT recipients.
Table 2.
Summary of Risk Factors
| Baseline characteristics | No. of articles | Total sample size | Effect size | P value |
|---|---|---|---|---|
| Age group | ||||
| 26–50 y old | 15 | 1836 | OR: 0.92 (CI: 0.76–1.13) | .46 |
| Above 50 y old | 24 | 2585 | OR: 1.01 (CI: 0.93–1.09) | .85 |
| Gender | ||||
| Male | 46 | 3109 | OR: 1.22 (CI: 1.01–1.48) | .088 |
| Female | 46 | 1944 | OR: 0.82 (CI: 0.68–0.99) | .088 |
| Indication for transplant | ||||
| HBV | 12 | 1131 | OR: 0.95 (CI: 0.87–1.04) | .27 |
| HCV | 18 | 1814 | OR: 1.06 (CI: 0.86–1.30) | .61 |
| Alcoholic liver | 24 | 2358 | OR: 1.14 (CI: 1.10–1.18) | <.001a |
| NASH | 7 | 908 | OR: 1.38 (CI: 0.55–3.48) | .53 |
| Ethnicity | ||||
| Caucasian | 12 | 1186 | OR: 1.42 (CI: 0.99–2.04) | .14 |
| Hispanic | 4 | 508 | OR: 0.015 (CI: 0.00011–2.21) | .10 |
| African-American | 6 | 723 | OR: 1.35 (CI: 0.53–3.45) | .63 |
| Education | ||||
| Elementary | 15 | 2055 | OR: 0.85 (CI: 0.67–1.08) | .21 |
| High school | 13 | 1932 | OR: 1.04 (CI: 0.80–1.36) | .77 |
| College | 5 | 492 | OR: 0.47 (CI: 0.23–0.97) | .14 |
| University | 5 | 584 | OR: 0.34 (CI: 0.17–0.67) | .051a |
| Employment status | ||||
| Employed | 26 | 3087 | OR: 1.13 (CI: 0.83–1.54) | .44 |
| Marital status | ||||
| Divorced | 5 | 558 | OR: 1.00 (CI: 0.54–1.82) | .99 |
| Stable relationship | 24 | 2886 | OR: 1.05 (CI: 0.78–1.41) | .77 |
CI, confidence interval; HBV, hepatitis B; HCV, hepatitis C; NASH, nonalcoholic steatohepatitis; OR, odds ratio.
P < .05 is statistically significant.
Outcomes of Depression
Odds of mortality for LT recipients with depression was 1.82 (CI: 1.08–3.07, P = .04, n = 328) compared with nondepressed patients. Patients with depression also had increased likelihood of graft loss (OR: 2.64, CI: 1.64–4.24, P = .03, n = 664) and graft rejection (OR: 1.76, CI: 1.12–2.77, P = .01, n = 470). However, no significant association was found between depression and alcohol consumption after LT (OR: 1.56, CI: 0.76–3.19, P = .44, n = 384; Table 3).
Table 3.
Summary of LT Outcomes
| Outcomes | No. of articles | Total sample size | Events | Effect size | P value |
|---|---|---|---|---|---|
| Mortality7,23 | 2 | 328 | 104 | OR: 1.82 (CI: 1.08–3.07) | .04a |
| Alcohol consumption after LT7,24 | 2 | 384 | 126 | OR: 1.56 (CI: 0.76–3.19) | .44 |
| Graft loss5,7,23,25 | 4 | 664 | 103 | OR: 2.64 (CI: 1.64–4.24) | .03a |
| Graft rejection5,25,26 | 3 | 470 | 124 | OR: 1.76 (CI: 1.12–2.77) | .01a |
CI, confidence interval; LT, liver transplantation; OR, odds ratio.
P < .05 is statistically significant.
Discussion
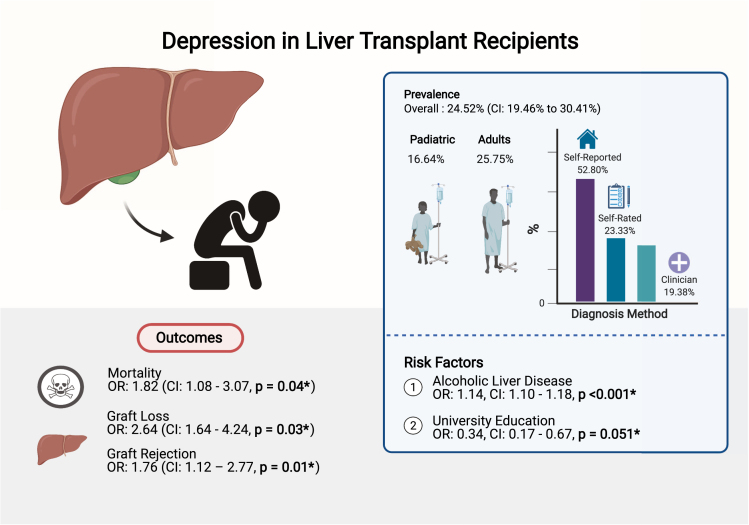
Previous reviews have focused on assessing the effects of LT on general QOL including physical functioning and mental wellbeing (Supplementary Material 4), but robust evidence on pooled prevalence estimates and predictors of depression in the LT population remains lacking. This meta-analysis of 48 articles synthesizes the global prevalence, risk factors, and outcomes of depression among 5170 LT recipients (Figure 3). In this study, we found that depression was highly prevalent after LT affecting nearly one in 4 LT recipients and was less common as follow-up duration increased, consistent with the trend towards improved mental health with more years after transplantation.27
Figure 3.
Summary of prevalence, risk factors, and outcomes of depression in LT recipients. ∗P ≤ .05 denotes statistical significance.
In this study, depression in LT recipients was associated with increased mortality, graft loss, and graft rejection. For all included studies, a diagnosis of depression preceded the post-LT outcome of interest. While it is unclear how graft rejection and failure can be affected, one possible behavioral mechanism may be the decreased adherence to post-transplant immunosuppressive medication regimen among patients with depression.28 Patients with depression may be less willing to participate in essential clinical care such as cancer screening or cardiovascular risk assessment, which can negatively affect survival in a population at significantly higher mortality risk from these. Moreover, depressed individuals often experience social isolation and lack of social support, both of which are established predictors of mortality.29,30
Previous reviews have also shown that depression contributes to hazardous health behaviors,31 including substance use, which bodes poorly for long-term graft function.32 However, similar to the findings of Chuncharunee et al,33 our analysis found that depressed patients were not more likely to consume alcohol after LT. Concerningly, Errichiello et al34 also found that patients with major depression displayed significantly higher suicidal ideation (P ≤ .001), while 3 other included studies reported suicide attempts or ideation by recipients with depression.35, 36, 37 Notwithstanding, these findings should be interpreted with caution given the limited sample size in the analysis of the outcomes. More high-quality studies are needed to validate the adverse impacts of depression on clinical endpoints among LT recipients.
Recipients from Asia had the highest rates of depression, while the lowest rates were reported in the Middle Eastern region. The actual prevalence of depression in Asia is likely to be underreported as stigma around mental health issues may often result in lower utilization of mental health services.38 In addition, only a small minority of included studies originated from Asia (10.20%) compared with Europe (40.82%) and North America (31.25%). Furthermore, traditional screening methods for depression have also been demonstrated to be less sensitive in Asians.39 This suggests a dire need for more Asian studies to be conducted, given the lack of studies from this region despite the high depression rates.
In the analysis of factors associated with a depression diagnosis, university education was found to be protective while ALD increased depression risk in recipients. University-educated recipients were less likely to have depressive symptoms attributable to better resources and coping mechanisms with lower education levels being a commonly cited factor for higher depression rates.40,41 In contrast, patients transplanted for ALD had an increased likelihood of depression. The association of ALD with affective disorders, particularly major depression and neurotic disorders, is well recognised.42 A study of 6050 individuals by Hasin and Grant found a strong and specific association between prior alcohol dependence and risk of major depressive disorder.43 This is clinically significant as alcohol use disorder is the second most common indication for LT in the United States and Europe.44 Although emerging evidence suggests that other chronic liver diseases such as hepatitis C and non-alcoholic fatty liver disease may also increase risks of depression,45,46 no significant association was found in our pooled analysis of the existing literature. Interestingly, female LT recipients were not at increased risks of depression compared with their male counterparts. Although higher depression rates have been reported among women undergoing heart transplant,47 a study by Doering et al48 observed that depressive symptoms improved over time, and gender differences were no longer significant by 9 months, potentially explaining the nonsignificant results. Regardless, the contribution of gender to depressive symptomology of LT recipients deserves further attention, especially since heightened risk of depression is well-established in healthy female cohorts.49 Mechanisms underlying determinants of emotional distress among women recipients need to be empirically examined, and until then, the utility of gender to guide screening among LT recipients remains uncertain.
In this study, depression rates were highest with self-reporting compared with clinician or self-rated assessment tools. However, reliance on patients’ self-identification of depression may overestimate actual rates as symptoms of chronic liver disease such as fatigue, lack of appetite, and weight changes may easily be mistaken for somatic symptoms of depression.50 While self-rated questionnaires are often used in clinical settings for screening or clinical research because of the speed and ease of administration, it is important to note that clinician-rated diagnosis remains the gold standard.50 BDI and HADS depression scores were most commonly used with depression rates of 21.24% and 18.73%, respectively, in keeping with a previous review which showed strong correlation of these 2 instruments with clinician-assessed depression severity, establishing criterion validity.51 To date, there is still no validated disease-specific instruments to measure mental health disorders in LT population, which may explain the variation in prevalence of depression in this study. Besides developing more precise and standardized assessment tools to collect quantitative evidence, future research should also consider using qualitative analysis to yield insights into the complex nature of psychological challenges that LT recipients face.52
Implications on Practice
Transplant societies across the world recommend psychosocial assessment to ensure the psychological fitness of transplant candidates and to identify potential risk factors for nonadherence after LT.53, 54, 55 Moving forward, depression screening in LT recipients using validated assessment instruments, especially for recipients transplanted for ALD, older individuals, and those with lower education level, could potentially be emphasized as a crucial aspect of psychosocial evaluations. Previous reviews have also recommended the treatment of depression in transplant patients with medication and psychotherapy, with caution given to the hepatotoxicity of antidepressants and its drug interactions with immunosuppressants.56
Strengths and Limitations
This study has several strengths. To our knowledge, this is the first meta-analysis that systematically reviews the evidence of depression in LT recipients with a combined sample size of 5170 recipients. However, there remains much heterogeneity in the diagnosis method of depression and the cutoff values to define depression for each diagnostic criterion. While self-rated questionnaires measure symptoms rather than the actual disorders, which can only be established via a structured psychiatric or psychological interview, it is important to note that these proxies are practical, often used, and widely accepted in most well-established, reputable studies.19,57,58 In addition, I2, a measure of heterogeneity, was significantly large in this analysis albeit attributable to the large sample size. It is well recognized that large sample sizes often result in inflated heterogeneity estimates.59,60 As such, previous prevalence meta-analyses often yield a large I2 of >90%.61,62 This may suggest a lack of an appropriate tool for accurate measurements of heterogeneity. Instead, consistent with a previous review,19 multiple subgroup analyses were conducted to account for heterogeneity and to test for the robustness of associations. In the analysis of factors associated with depression diagnosis, we were unable to account for pre-LT psychiatric diagnosis and recipients’ relationship with donors and assess their living conditions because of a paucity of data. Similarly, outcomes including adherence to treatment and employment after LT were underreported. A possible confounder in the outcome analysis is that patients who suffered increased post-LT complications may have developed depression which further exacerbated clinical outcomes. These findings should also be interpreted with caution as the small sample size may have limited statistical power. Finally, further stratification of risk factors and outcomes by pediatric population could not be conducted owing to a sparsity of data although depression rates were similar between adult and pediatric populations. Importantly, this study highlights common risk factors of depression in LT recipients and consolidates clinically important transplant-related outcomes.
Conclusion
This systematic review highlights the substantial psychological burden among LT recipients, especially among recipients transplanted for ALD and those with lower education levels. Development of integrated models of care that encompass holistic management of clinical endpoints and psychological comorbidity is key to quality patient care. More high-quality studies are warranted to validate the adverse effects of depression on clinical outcomes in LT recipients including increased mortality risk and poorer graft outcomes.
Acknowledgments:
All authors have made substantial contributions to all the following steps in the study: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures, and tables, has not been previously published, and the manuscript is not under consideration elsewhere.
Authors' Contributions:
Conceptualization: Wen Hui Lim, Cheng Han Ng, Mohammad Shadab Siddiqui, and Mark Dhinesh Muthiah. Data curation: Wen Hui Lim, Chen Wei Poh, Beatrice Jia Min Tan, Cheng Han Ng, Darren Jun Hao Tan, Xiong Chang Lim, Phoebe Wen Lin Tay, and Grace En Hui Lim. Formal analysis: Wen Hui Lim, Chen Wei Poh, Beatrice Jia Min Tan, Cheng Han Ng, and Nicholas Syn. Supervision: Daniel Q. Huang, Cyrus S.H. Ho, Eunice Xiang-Xuan Tan, Nicholas Syn, Yock Young Dan, Konstadina Griva, James Fung, Mohammad Shadab Siddiqui, and Mark Dhinesh Muthiah. Validation: Wen Hui Lim, Cheng Han Ng, Darren Jun Hao Tan, Xiong Chang Lim, Daniel Q. Huang, Cyrus S.H. Ho, Eunice Xiang-Xuan Tan, and Nicholas Syn. Writing the original draft: Wen Hui Lim, Chen Wei Poh, Beatrice Jia Min Tan, and Cheng Han Ng. Writing, reviewing, and editing: Wen Hui Lim, Chen Wei Poh, Beatrice Jia Min Tan, Cheng Han Ng, Darren Jun Hao Tan, Xiong Chang Lim, Phoebe Wen Lin Tay, Grace En Hui Lim, Daniel Q. Huang, Cyrus S.H. Ho, Eunice Xiang-Xuan Tan, Nicholas Syn, Yock Young Dan, Konstadina Griva, James Fung, Mohammad Shadab Siddiqui, and Mark Dhinesh Muthiah. All authors have read and approved the final version of the manuscript for submission.
Footnotes
Conflicts of Interest: None of the authors declare any conflict of interest.
Funding: No grants or external funding was received for this study.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All articles in this manuscript are publicly available from Medline and Embase.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2021.12.001.
Contributor Information
Wen Hui Lim, Email: whlim0403@gmail.com.
Mark Dhinesh Muthiah, Email: mdcmdm@nus.edu.sg.
Supplementary Materials
References
- 1.Yang L.S., Shan L.L., Saxena A., et al. Liver transplantation: a systematic review of long-term quality of life. Liver Int. 2014;34:1298–1313. doi: 10.1111/liv.12553. [DOI] [PubMed] [Google Scholar]
- 2.Dew M.A., Rosenberger E.M., Myaskovsky L., et al. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: a systematic review and meta-analysis. Transplantation. 2016;100:988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogal S., Shenai N., Kruckenberg K., et al. Post-transplant outcomes of persons receiving a liver graft for alcoholic liver disease. Alcohol Alcohol. 2018;53:157–165. doi: 10.1093/alcalc/agx100. [DOI] [PubMed] [Google Scholar]
- 4.Annema C., Roodbol P.F., Stewart R.E., et al. Prevalence of psychological problems and associated transplant-related variables at different time periods after liver transplantation. Liver Transpl. 2015;21:524–538. doi: 10.1002/lt.24075. [DOI] [PubMed] [Google Scholar]
- 5.Annema C., Drent G., Roodbol P.F., et al. Trajectories of anxiety and depression after liver transplantation as related to outcomes during 2-year follow-up: a prospective cohort study. Psychosom Med. 2018;80:174–183. doi: 10.1097/PSY.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 6.Mullish B.H., Kabir M.S., Thursz M.R., et al. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther. 2014;40:880–892. doi: 10.1111/apt.12925. [DOI] [PubMed] [Google Scholar]
- 7.Rogal S.S., Dew M.A., Fontes P., et al. Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant. 2013;13:928–935. doi: 10.1111/ajt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar A., Vandriel S.M., Ng V.L. Health-related quality of life after pediatric liver transplantation: a systematic review. Liver Transpl. 2017;23:361–374. doi: 10.1002/lt.24696. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo J.H. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38:171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail Z., Elbayoumi H., Fischer C.E., et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:58–67. doi: 10.1001/jamapsychiatry.2016.3162. [DOI] [PubMed] [Google Scholar]
- 12.Wagner S., Wollschläger D., Dreimüller N., et al. Effects of age on depressive symptomatology and response to antidepressant treatment in patients with major depressive disorder aged 18 to 65 years. Compr Psychiatry. 2020;99:152170. doi: 10.1016/j.comppsych.2020.152170. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y., Zhu Q., Wang B., et al. A cross-sectional study on interference control: age affects reactive control but not proactive control. PeerJ. 2020;8 doi: 10.7717/peerj.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell A., Fairbrother M., Jones K. Fixed and random effects models: making an informed choice. Qual Quant. 2019;53:1051–1074. [Google Scholar]
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Borges Migliavaca C., Stein C., Colpani V., et al. How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol. 2020;20:96. doi: 10.1186/s12874-020-00975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Q., Zou B., Yeo Y.H., et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 18.Huang D.Q., Yeo Y.H., Tan E., et al. ALT levels for Asians with metabolic diseases: a meta-analysis of 86 studies with individual patient data validation. Hepatol Commun. 2020;4:1624–1636. doi: 10.1002/hep4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barberio B., Zamani M., Black C.J., et al. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359–370. doi: 10.1016/S2468-1253(21)00014-5. [DOI] [PubMed] [Google Scholar]
- 20.Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011;31:1117–1125. doi: 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Harris R., Bradburn M., Deeks J., et al. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 22.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Sebaaly J.C., Fleming J., Pilch N., et al. Depression, resource utilization, and outcomes following liver transplant. Prog Transplant. 2016;26:270–276. doi: 10.1177/1526924816654641. [DOI] [PubMed] [Google Scholar]
- 24.Koljonen V., Åberg F., Rovasalo A., et al. Self-reported alcohol use and depressive symptoms after liver transplantation. Transplantation. 2015;99:867–872. doi: 10.1097/TP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 25.Nickel R., Wunsch A., Egle U.T., et al. The relevance of anxiety, depression, and coping in patients after liver transplantation. Liver Transpl. 2002;8:63–71. doi: 10.1053/jlts.2002.30332. [DOI] [PubMed] [Google Scholar]
- 26.Corruble E., Barry C., Varescon I., et al. Depressive symptoms predict long-term mortality after liver transplantation. J Psychosom Res. 2011;71:32–37. doi: 10.1016/j.jpsychores.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Karam V.H., Gasquet I., Delvart V., et al. Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation. 2003;76:1699–1704. doi: 10.1097/01.TP.0000092955.28529.1E. [DOI] [PubMed] [Google Scholar]
- 28.Burra P., Germani G., Gnoato F., et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17:760–770. doi: 10.1002/lt.22294. [DOI] [PubMed] [Google Scholar]
- 29.Holt-Lunstad J., Smith T.B., Layton J.B. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantell M., Rehkopf D., Jutte D., et al. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health. 2013;103:2056–2062. doi: 10.2105/AJPH.2013.301261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuijpers P., Schoevers R.A. Increased mortality in depressive disorders: a review. Curr Psychiatry Rep. 2004;6:430–437. doi: 10.1007/s11920-004-0007-y. [DOI] [PubMed] [Google Scholar]
- 32.Dew M.A., DiMartini A.F., Steel J., et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl. 2008;14:159–172. doi: 10.1002/lt.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuncharunee L., Yamashiki N., Thakkinstian A., et al. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:150. doi: 10.1186/s12876-019-1050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Errichiello L., Picozzi D., de Notaris E.B. Prevalence of psychiatric disorders and suicidal ideation in liver transplanted patients: a cross-sectional study. Clin Res Hepatol Gastroenterol. 2014;38:55–62. doi: 10.1016/j.clinre.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Everson G., Bharadhwaj G., House R., et al. Long-term follow-up of patients with alcoholic liver disease who underwent hepatic transplantation. Liver Transpl Surg. 1997;3:263–274. doi: 10.1002/lt.500030312. [DOI] [PubMed] [Google Scholar]
- 36.Ruth N., Sharif K., Legarda M., et al. What is the long-term outlook for young people following liver transplant? A single-centre retrospective analysis of physical and psychosocial outcomes. Pediatr Transplant. 2020;24:e13782. doi: 10.1111/petr.13782. [DOI] [PubMed] [Google Scholar]
- 37.Hames A., Matcham F., Joshi D., et al. Liver transplantation and adolescence: the role of mental health. Liver Transpl. 2016;22:1544–1553. doi: 10.1002/lt.24629. [DOI] [PubMed] [Google Scholar]
- 38.Augsberger A., Yeung A., Dougher M., et al. Factors influencing the underutilization of mental health services among Asian American women with a history of depression and suicide. BMC Health Serv Res. 2015;15:542. doi: 10.1186/s12913-015-1191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comino E., Silove D., Manicavasagar V., et al. Agreement in symptoms of anxiety and depression between patients and GPs: the influence of ethnicity. Fam Pract. 2001;18:71–77. doi: 10.1093/fampra/18.1.71. [DOI] [PubMed] [Google Scholar]
- 40.Peyrot W.J., Lee S.H., Milaneschi Y., et al. The association between lower educational attainment and depression owing to shared genetic effects? Results in ∼25000 subjects. Mol Psychiatry. 2015;20:735–743. doi: 10.1038/mp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng C.H., Lim W.H., Lim X.C., et al. A meta-analysis on the incidence of donor-related depression after liver transplant. Transpl Int. 2021;34:2061–2070. doi: 10.1111/tri.13975. [DOI] [PubMed] [Google Scholar]
- 42.Ewusi-Mensah I., Saunders J.B., Wodak A.D., et al. Psychiatric morbidity in patients with alcoholic liver disease. Br Med J (Clin Res Ed) 1983;287:1417–1419. doi: 10.1136/bmj.287.6403.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasin D.S., Grant B.F. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- 44.Singal A.K., Guturu P., Hmoud B., et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. doi: 10.1097/TP.0b013e31827afb3a. [DOI] [PubMed] [Google Scholar]
- 45.Huang X., Liu X., Yu Y. Depression and chronic liver diseases: are there shared underlying mechanisms? Front Mol Neurosci. 2017;10:134. doi: 10.3389/fnmol.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao J., Lim L.K.E., Ng C.H., et al. Is fatty liver associated with depression? A meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front Med (Lausanne) 2021;8:691696. doi: 10.3389/fmed.2021.691696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evangelista L.S., Doering L., Dracup K. Meaning and life purpose: the perspectives of post-transplant women. Heart Lung. 2003;32:250–257. doi: 10.1016/s0147-9563(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 48.Doering L., Chen B., Hickey K., et al. Abstract 16052: gender differences in post-heart transplant trajectories of anxiety, depressive symptoms and health-related quality of life. Circulation. 2018;134 [Google Scholar]
- 49.Albert P.R. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40:219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirazian S., Grant C.D., Aina O., et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2016;2:94–107. doi: 10.1016/j.ekir.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jay C.L., Butt Z., Ladner D.P., et al. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong A., Chapman J.R., Israni A., et al. Qualitative research in organ transplantation: recent contributions to clinical care and policy. Am J Transplant. 2013;13:1390–1399. doi: 10.1111/ajt.12239. [DOI] [PubMed] [Google Scholar]
- 53.Miller C.M., Quintini C., Dhawan A., et al. The International Liver Transplantation Society living donor liver transplant recipient guideline. Transplantation. 2017;101:938–944. doi: 10.1097/TP.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin P., DiMartini A., Feng S., et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 55.EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Surman O.S., Cosimi A.B., DiMartini A. Psychiatric care of patients undergoing organ transplantation. Transplantation. 2009;87:1753–1761. doi: 10.1097/TP.0b013e3181a754d4. [DOI] [PubMed] [Google Scholar]
- 57.Fancourt D., Steptoe A., Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. Lancet Psychiatry. 2021;8:141–149. doi: 10.1016/S2215-0366(20)30482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karyotaki E., Efthimiou O., Miguel C., et al. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry. 2021;78:361–371. doi: 10.1001/jamapsychiatry.2020.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 60.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borenstein M., Higgins J.P., Hedges L.V., et al. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 62.Rücker G., Schwarzer G., Carpenter J.R., et al. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.