Since the inception of balloon angioplasty, restenosis has been the Achilles heel of percutaneous coronary intervention (PCI), with many early years spent in futility trying to find a magic pill or plaque-removing device to prevent it. Seventeen years after Gruentzig performed the initial angioplasty, the first major breakthrough to reduce restenosis came with the landmark STRESS and BENESTENT trials. In the STRESS trial, angiographic restenosis was reduced from 42.1% after balloon angioplasty to 31.6% with the Palmaz-Schatz stent.1 Although bare metal stents (BMS) reduced restenosis due to their scaffolding effect, paradoxically postprocedural late lumen loss was substantially greater because of the proliferation of neointima within the stent. Thus, with the introduction of BMS, the Achilles tendon, although no longer completely severed, remained severely stretched.
The solution to the problem of neointimal proliferation came with the idea of using drugs in a time-released capsule, not as a pill but as a coating on the stent. Thus, the ultimate weapon against restenosis, the drug-eluting stent (DES), was born. Although first-generation DES were fraught with problems of stent thrombosis, clinical outcomes have continued to improve with newer-generation DES owing to technological advances such as thinner stent struts and more biocompatible polymers. In turn, PCI is increasingly undertaken in patients who are at higher risk with more complex anatomy, so the problem of restenosis has far from faded. In the RESOLUTE All-Comers trial of 2292 patients who underwent PCI without restrictions, the target vessel revascularization (TVR) rate at 2 years with new-generation DES was approximately 10%.2
In patients with clinical restenosis of DES, is there a preferred strategy? Based on results from meta-analyses, the recent ACC/AHA/SCAI revascularization guidelines give a class I recommendation for PCI using a second DES3; however, use of an overlapped second DES carries a higher risk of in-stent restenosis (ISR) than that for de novo procedures. Approximately 10% to 20% of patients treated for ISR with 2 overlapping layers of DES will require a third TVR procedure. For patients in whom recurrent DES restenosis develops, the outcome with further PCI is poor. In our center, of patients undergoing a third PCI for recurrent DES restenosis, only 24.5% remained free of a major cardiac event and 63.5% underwent a fourth TVR after 3 years.4 Similarly, Theodoropoulos et al5 reported a target lesion failure (TLF) rate of 51% at 2 years irrespective of whether the recurrent restenosis was treated by balloon angioplasty or additional DES. Thus, performing a third PCI with conventional techniques is likely to fail and conforms to Einstein’s definition of insanity—“Doing the same thing over and over and expecting different results.” Accordingly, alternative approaches such as coronary artery bypass surgery should be considered. A percutaneous option frequently used abroad is angioplasty with drug-eluting balloons, which are currently not available in the United States for coronary arteries.
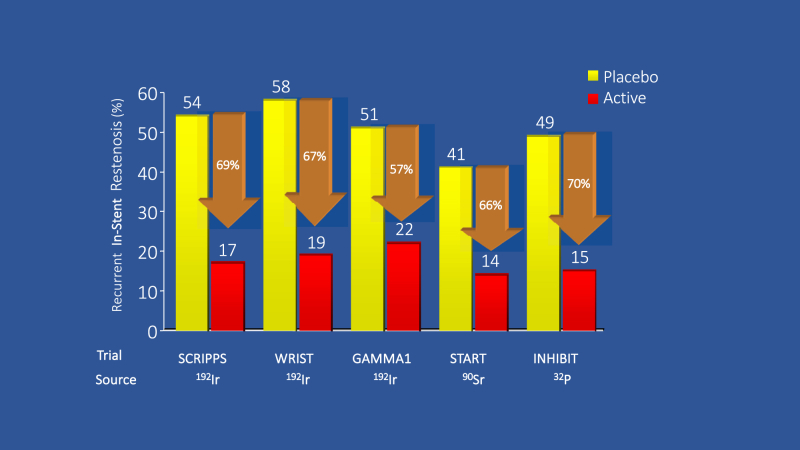
The absence of effective options for the vexing problem of incessant restenosis has led to the resurgence of a technique associated with the BMS era—intracoronary brachytherapy. Before the introduction of DES, coronary brachytherapy was the default treatment for ISR. Performed at the time of repeat angioplasty, the localized delivery of radiation was highly effective in prohibiting the regrowth of neointima by causing apoptosis of vascular smooth muscle cells and myofibroblasts. Several randomized trials demonstrated that intravascular brachytherapy reduced recurrent restenosis in BMS by two-thirds (Figure 1).
Figure 1.
Randomized controlled trials of intravascular brachytherapy to treat restenosis of bare metal stents. Intracoronary brachytherapy resulted in a median 67% relative reduction in recurrent in-stent restenosis.
Approved by the US Food and Drug Administration on November 3, 2000, the Novoste Beta-Cath intravascular brachytherapy system (Best Vascular, Inc) was rapidly adopted. Within a short time span, the device was in use at 509 US centers; however, the golden age of coronary brachytherapy was relatively short-lived because the first DES received US Food and Drug Administration approval in April 2003. After the approval of DES, utilization of coronary brachytherapy dwindled. Not only were the number of patients with restenosis significantly fewer but, more importantly, randomized trials demonstrated that first-generation DES were more effective in treating BMS restenosis than brachytherapy.6,7 As a consequence, most centers discontinued their brachytherapy programs. By 2012, the number of institutions performing coronary brachytherapy dropped by >96% nationwide to a mere 18 centers. The procedure is no longer performed outside of the United States.
As the DES era has matured, it has become clear that although diminished, restenosis has not disappeared. For patients with a “DES sandwich” who have recurrent restenosis, the outcomes with further PCI procedures are dismal. The absence of effective options for these patients has spurred a renewed interest in coronary brachytherapy. In recent years, the number of active coronary brachytherapy sites has rebounded 133% to 42 centers (J. Craig Reed, BS, Best Vascular, Inc, oral and text communication, November 21, 2022). From 2009 to 2017, use of intravascular brachytherapy for ISR increased nearly 10-fold.8 ACC/AHA/SCAI revascularization guidelines now bestow coronary brachytherapy a class 2b recommendation for recurrent ISR.3
The efficacy of coronary brachytherapy in the seminal trials was demonstrated for BMS restenosis with single stents (Figure 1); however, coronary brachytherapy currently is most often used to treat recurrent ISR after 2 or more layers of overlapping DES. To date, there are no prospective, randomized trials of brachytherapy for DES restenosis.
In this issue of JSCAI, Ho et al9 report on one of the largest series of patients with DES restenosis treated with coronary brachytherapy. The Cleveland Clinic investigators studied 330 consecutive patients who underwent intracoronary brachytherapy for DES restenosis. The median number of recurrent ISR episodes was 3; recurrent ISR with ≥2 stent layers was present in 89%. Most of the patients presented with diabetes and previous coronary artery bypass graft surgery. TLF was 18% at 1 year and 46% at 3 years. The only significant predictor of TLF at 3 years was the number of stent layers: 33% with 1 layer, 47% with 2 layers, and 60% with ≥3 layers. TLF was not associated with diabetes status, lesion length, or final percentage of stenosis. The low TLF at 1 year but high rate at 3 years suggests a late catch-up phenomenon after brachytherapy for DES ISR, as has been previously reported with BMS.10 Similar findings have been reported by others.11,12
The relatively high TLF rates at 3 years must be viewed in the context of TLF rates exceeding 50% without brachytherapy after a third PCI for recurrent ISR. Varghese et al13 studied 328 patients treated with repeat PCI for DES ISR with at least 2 stent layers. At 1 year, major adverse cardiovascular events occurred in 28.2% of 131 patients treated without brachytherapy compared with 13.2% of 197 patients treated with brachytherapy (P = .01). Follow-up beyond 1 year was not reported.
Observational investigations such as these are welcome additions to the literature because there are few studies on the outcomes of coronary brachytherapy in DES. The decremental benefit of brachytherapy with increasing stent layers has potential treatment implications. In patients with 3 or more stent layers, TLF was 60%. Potential mechanistic explanations include a shielding effect from multiple overlapping stent struts and hostile underlying vascular substrate. Similar findings have been reported with drug-eluting balloons in the treatment of recurrent DES restenosis where TLF is significantly higher for patients with ≥3 stent layers.14 These findings suggest that once recurrent ISR reoccurs after 2 overlapping DES, alternative therapies such as brachytherapy or drug-eluting balloons should be considered, whereas a third layer of stents can be counterproductive.
In conclusion, the demise of coronary restenosis has been greatly exaggerated. Recurrent restenosis after multiple DES presents a perplexing and frustrating clinical challenge. Intracoronary brachytherapy seems to be an attractive option, but it should be recognized that the evidence basis is nascent and soft. It is unlikely that any single weapon will win the war against refractory restenosis. It is more likely that a multifront attack will be necessary by combining the forces of intravascular imaging, brachytherapy, drug-eluting balloons, and future development of novel antiproliferative therapies.
Acknowledgments
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Fischman D.L., Leon M.B., Baim D.S., et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 2.Silber S., Windecker S., Vranckx P., Serruys P.W., RESOLUTE All Comers investigators Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet. 2011;377(9773):1241–1247. doi: 10.1016/S0140-6736(11)60395-4. [DOI] [PubMed] [Google Scholar]
- 3.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145(3):e18–e114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 4.Savage M.P., Luo M., Ravipati G., et al. Paper presented at: American College of Cardiology Scientific Session; Washington, DC: 2022. Three time loser or third time’s the charm? Outcome of repeated percutaneous coronary intervention for recurrent restenosis after two layers of drug-eluting stents. April 2. [Google Scholar]
- 5.Theodoropoulos K., Mennuni M.G., Dangas G.D., et al. Resistant in-stent restenosis in the drug eluting stent era. Catheter Cardiovasc Interv. 2016;88(5):777–785. doi: 10.1002/ccd.26559. [DOI] [PubMed] [Google Scholar]
- 6.Stone G.W., Ellis S.G., O’Saughnessy C.D., et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253–1263. doi: 10.1001/jama.295.11.1253. [DOI] [PubMed] [Google Scholar]
- 7.Holmes D.R., Teirstein P., Satler L., et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295(11):1264–1273. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 8.Moussa I.D., Mohananey D., Saucedo J., et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–1531. doi: 10.1016/j.jacc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Ho E., Cherian S., Ciezki J., et al. Intracoronary brachytherapy for drug-eluting stent restenosis: outcomes and clinical correlates. J Soc Cardiovasc Angiogr Interv. 2023;2(1):100550. [Google Scholar]
- 10.Waksman R., Ajani A.E., White R.L., et al. Five-year follow-up after intracoronary gamma radiation therapy for in-stent restenosis. Circulation. 2004;109(3):340–344. doi: 10.1161/01.CIR.0000109488.62415.01. [DOI] [PubMed] [Google Scholar]
- 11.Negi S.I., Torguson R., Gai J., et al. Intracoronary brachytherapy for recurrent drug-eluting stent failure. JACC Cardiovasc Interv. 2016;9(12):1259–1265. doi: 10.1016/j.jcin.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Mangione F.M., Jatene T., Eslam R.B., et al. Usefulness of intracoronary brachytherapy for patients with resistant drug-eluting stent restenosis. Am J Cardiol. 2017;120(3):369–373. doi: 10.1016/j.amjcard.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Varghese M.J., Bhatheja S., Baber U., et al. Intravascular brachytherapy for the management of repeated multimetal-layered drug-eluting coronary stent restenosis. Circ Cardiovasc Interv. 2018;11(10) doi: 10.1161/CIRCINTERVENTIONS.118.006832. [DOI] [PubMed] [Google Scholar]
- 14.Yabushita H., Kawamoto H., Fujino Y., et al. Clinical outcomes of drug-eluting balloon for in-stent restenosis based on the number of metallic layers. Circ Cardiovasc Interv. 2018;11(8) doi: 10.1161/CIRCINTERVENTIONS.117.005935. [DOI] [PubMed] [Google Scholar]